(download as a pdf - 3.4Mb)
The skin is the body’s largest organ. In a 70kg person it weighs over 5kg and covers a surface area of almost 2m2. It performs the functions of(1, 2):
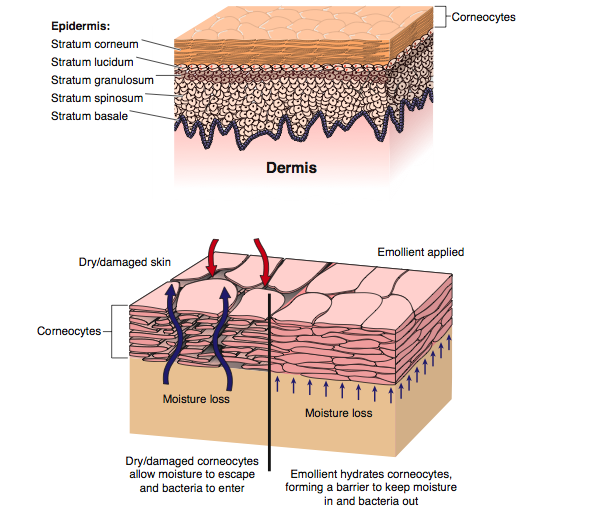
The barrier function of the skin relies on a well hydrated epidermis. It is also this level of moisture in the stratum corneum that helps to regulate the pliability and elasticity of the skin(3).
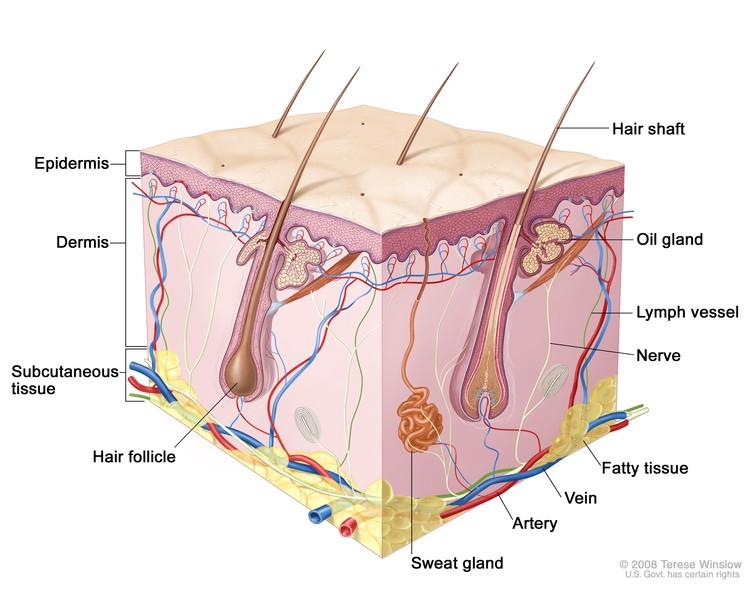
The skin has three layers, the thin epidermis which itself is composed of multiple layers, the thicker dermis and the hypodermis or what used to be referred to as subcutaneous tissue. The skin is constantly remodelling itself based on external stimuli. Cells are produced in the basal layer of the epidermis and migrate to the outer layers to eventually flake off. This process takes about 28-30 days but is accelerated in diseases like psoriasis(2, 4). Aging, illness, medications and the effects of sunlight on skin also impact on the anatomy of the skin and how it responds to environmental stresses and trauma(5, 6). A table of changes and their clinical implications can be seen in Appendix A.
Made up of connective tissue and adipose tissue this layer provides a support framework allowing for attachment to bones and muscle. The adipose tissue in this layer improves it’s ability to provide thermal insulation and cushioning from traumatic forces as well as being a store of energy reserves(4, 7).
This is a layer of collagen and elastin that provides a support framework for nerve endings, blood vessels, lymphatic capillaries, sweat glands, sebaceous glands and hair follicles. It ranges from 0.5 to 5mm thick. It can be divided in to the papillary layer (the undulating interface with the epidermis) and reticular layer (deeper dermis, is more dense, houses blood supply and adnexals). The superficial venus plexus and deep venus plexus form a network of capillary beds responsible for heat regulation. Lymphatics follow the blood vessels, becoming larger as they go deeper. Innervation of the skin is via the autonomic nervous system affecting things like sweating and flushing. Sensory fibres register painful stimuli and there are a number of nerve receptors (transducers) that register touch, pressure and vibration(2, 8).
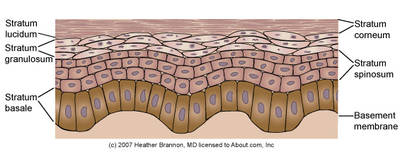
The epidermis ranges from 0.05 to 0.1mm thick depending on sex and anatomic location. The blood supply in the dermis does not pass the dermal/epidermal boundary so the epidermis is avascular. Instead, proteins and other nutrients are diffused through the thin basement membrane of the epidermis from the capillary beds of the dermis(2, 9). The epidermis is composed of five layers:
This is the layer at the dermal/epidermal boundary. It is a continuous layer, one cell thick but may be two to three cells thick in non-hairy (glabrous) skin. Here keratinocytes divide and begin differentiation. Epidermal ridges interlock with dermal ridges (rete pegs and dermal papillae) forming the dermal/epidermal junction(4).
This layer gets its name from spiny projections, of desmosomes, which are points of strong adhesion between keratinocytes. Where desmosomes are not present or functioning the adhesion between the cells is broken and the result is either skin loss or the presence of blistering depending on the extent of the damage to the desmosomes. Examples include genetic malformation of the desmosomes (epidermolysis bullosa), infectious damage (bullous impetigo) and autoimmune disruption (pemphigus)(2, 9). Langerhans’ Cells are found mainly in this layer(3). Langerhans cells are a part of the body’s immune system. These cells can interact with lymphocytes, are able to process and present antigens and are capable of phagocytosis. They can also release cytokines, to attract and activate lymphocytes. Studies in mice have also shown that Langerhan cells regulate the balance between immunity and peripheral tolerance; mice without these cells had hypersensitive skin(2).
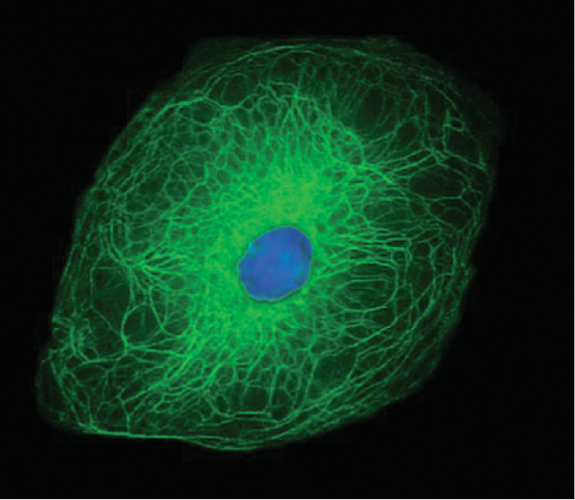
The cells that were still actively deviding in the previous layer now start to flatten and lose their organelles as they progress through this layer(9). These cells contain granules of keratohyalin and lamellar bodies. The keratohyalin granules release filaggrin to form macrofibres. The image on the right shows the structural organization of the keratin filament network within a keratinocyte. Lamellar bodies are organelles containing waxy (ceremides) and fatty (sterols and fatty acids) compounds. These organelles migrate to the edge of the cell, fuse with the plasma membrane and disperse their contents into the intercellular space. This plays an important role in barrier function and intercellular cohesion(2). The resulting extracellular lipids are sometimes referred to as the acid mantle. In atopic dermatitis (AD) the lamellar bodies are blocked from releasing their contents, resulting in the poor barrier function of the skin in AD(10).
In palmoplantar skin an extra layer of cells is present. These are still nucleated and may be considered transitional(2).
The outermost layer is composed of layers of non-nucleated, cornified keratinocytes. This layer is resistant to pH changes, temperature and dehydration(2). Around the early 1980’s it was first proposed that the outer layer of the skin had a bricks-and-mortar style arrangement. The bricks represent the keratinocytes; the now dead, flattened, and hardened with keratin, cells. And the mortar is a lipid bilayer which started out as basic sterols and waxes secreted by the lamellar bodies into the extracellular space and are now part of a structured environment which block the outflow of water into the environment and prevent the ingress of toxic substances, allergens, and microbial pathogens into the body. The lipid bilayer accounts for approximately 10% of the mass of the stratum corneum(10).
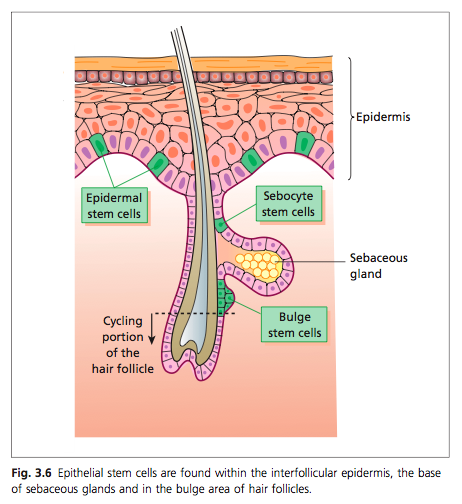
AKA epithelial appendages are important for the re-epithelialization where a wound has occurred(9). Epithelial stem cells can be found in the basal layer, follicular epidermis, base of sebaceous glands and the bulge area(2).
Hair is also derived from keratin. A pocket of epithelium forms which is continuous with the epidermis. This pocket encloses:
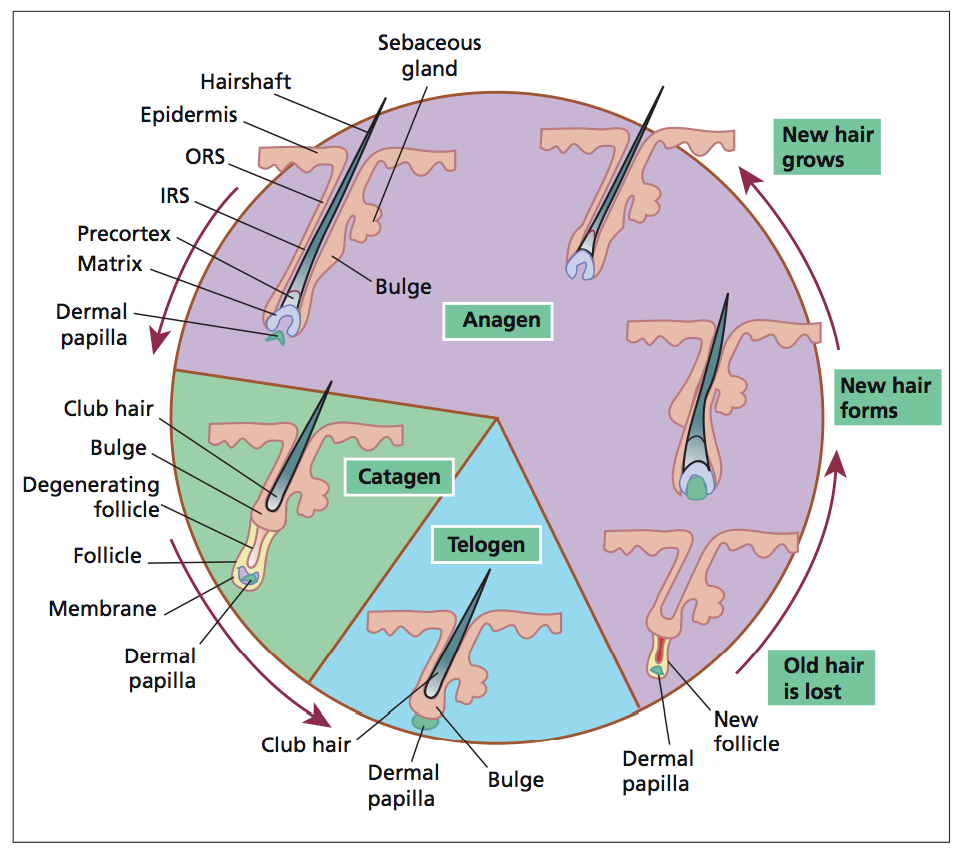
There are three phases of hair growth:
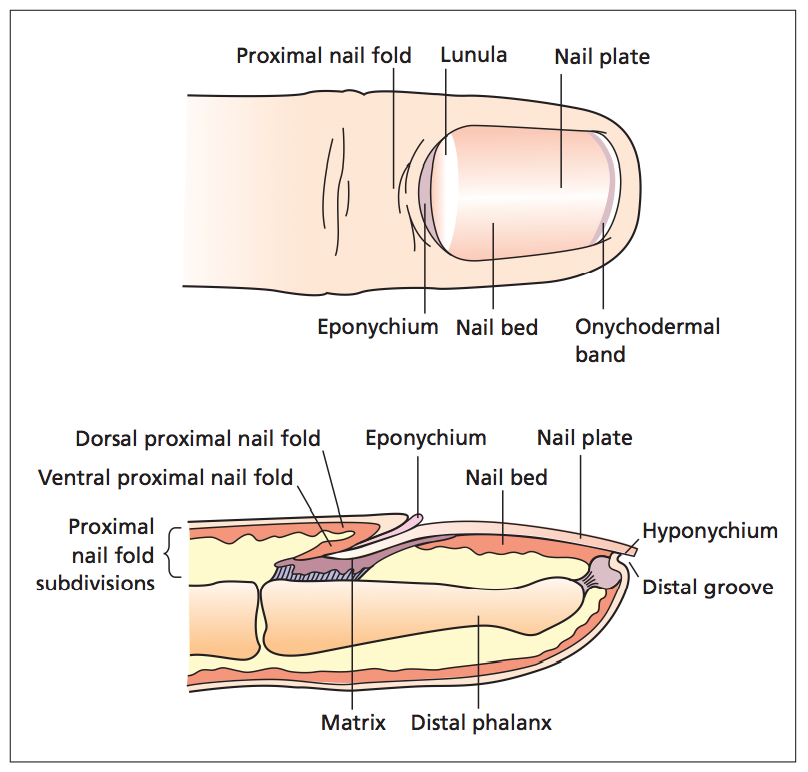
The main purpose of the nail is for protection of the digit tip, enhance sensory discrimination, increase dexterity and facilitate scratching or grooming. Some individuals also consider them to have a cosmetic function. This process by which the nail is made is similar to that of the stratum corneum. Instead of keratohyalin forming keratinocytes (to give it the skin it’s characteristics) the epidermal nail cells differentiate into onychocytes which contain calcium, phosphate, iron, zinc, manganese and copper, but it is mainly sulphur within the nail matrix that is responsible for the nail plate’s physical qualities(2, 11).
The nail is formed in the matrix epithelium, it is composed of at least two to three actively dividing, basal keratinocyte layers. The nail plate develops in an upward and outward direction, the cells become flatter, losing their nuclei and become onychocytes. The epidermis of the nail bed also contains parallel longitudinal ridges from the lunula (the visible portion of the distal matrix) to the hyponychium. These ridges interlock to provide strong binding between the nail bed and the nail plate. The dermal layer of the nail bed has lymphatics and an enhanced vasculature that supplies the nail unit providing its pink colour. Trauma to these vessels results in splinter haemorrhages(2, 11).
Apocrine glands are always connected to a hair follicle and can be found in genital, axil- lary and mammary areas. These glands have a low secretory output and do not play a significant role in thermoregulation. The lipid rich secretions are emptied into the hair follicle. The exact role of the apocrine glands in humans is not understood(2).
Sebaceous, or oil, glands produce lipid-rich sebum, which prevents transepidermal water loss and has antimicrobial properties, inhibiting growth of certain fungi and bacteria. Sebum production peaks in adolescence, providing an environment in which Propionibacterium acnes flourishes. Thereafter production decreases by 23% per decade in men and 32% per decade in women. Although sebaceous gland function diminishes with age, sebaceous gland size increases(5, 12).
Dendritic cells which capture antigen and present to T-Cells and Macrophages. Langerhans’ Cells are found mainly in the statum spinosum and are a part of the body’s immune system(3). These cells can interact with lymphocytes, are able to process and present antigens and are capable of phagocytosis. They can also release cytokines, to attract and activate lymphocytes. Studies in mice have also shown that Langerhan cells regulate the balance between immunity and peripheral tolerance; mice without these cells had hypersensitive skin(2).
Melanocytes are dendritic, pigment-producing cells located in the skin and hair, and other locations like the iris of the eye. The pigment they produce is called melanin which the melanocyte transfers in packets to neighbouring keratinocytes in the epidermis and into the growing shaft in hair follicles. Melanin production also provides a certain degree of protection from harmful ultraviolet radiation. Melanocyte density decreases with age by 6% to 8% per decade from age 30, and the melanocytes do not produce melanin pigment as efficiently. Melanocytes are still present in the hair follicles of people with gray hair, but melanin production is diminished here as it is in the epidermis(2, 5, 9).
Merkel cells are though to be mechanoreceptors which communicate with the afferent nerve conduction system in the basal layer in order to perceive the sensation of touch. Other transducer cell types are thought to be mechanoreceptors which work along side the merkel cells to detect vibration, touch and pressure. These are Ruffini organs, Meissner’s corpuscles, and Vater–Pacini corpuscles(9).
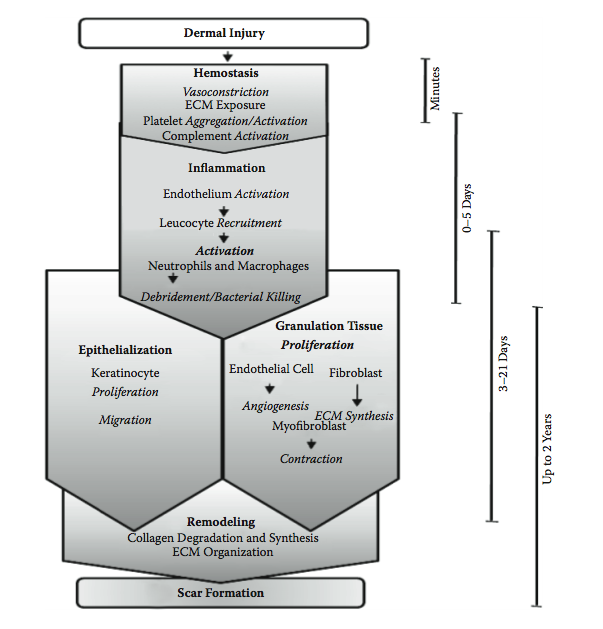
When a wounding to the skin occurs the body immediately responds to attempt to restore the normal function. The response involves stopping the bleeding, cleaning up the area and fighting off infection, re-establishing a suitable blood supply to the area to be able to make repairs, and repairing the damage. The result is not the regeneration of normal skin but a repair in the form of a scar(13).
Immediately after the injury, the damaged blood and lymphatic vessels undergo vasoconstriction to slow or stop blood loss in the affected area. Under normal conditions platelets circulate freely, they do not interact with intact vasculature. But in an injury they come into contact with distrurbed vasculature or extracellular matrix (ECM), this makes them adherent. Once they adhere they break open (degranulate) and release clotting factors and essential growth factors and cytokines. These cytokines attract leukocytes, fibroblasts and keratinocytes. Haemostasis is achieved by formation of a platelet-fibrin plug and the wound space fills with a fibrin clot. The clot provides temporary cover for the wound and acts as a provisional matrix that supports cell migration during healing(9, 14).
The growth factors and cytokines released by the platelets are important triggers not only for hemostasis, but as important messages for the later phases of healing. The coagulation cascade is needed to initiate wound healing(9).
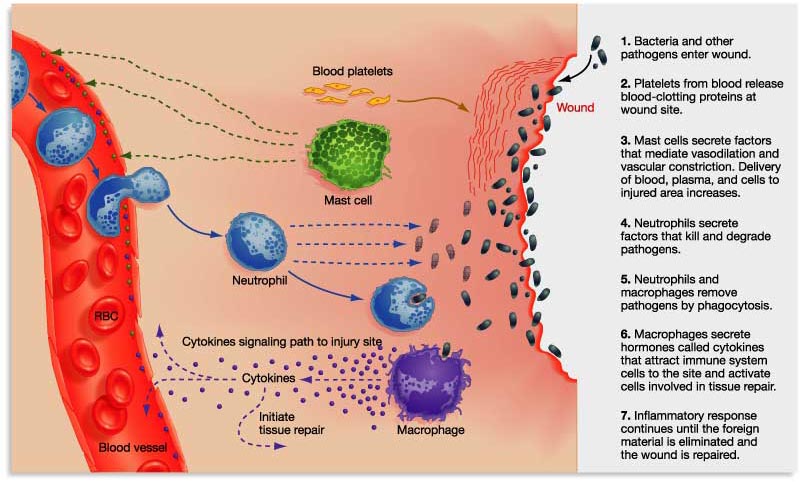
Neutrophils quickly enter the wound site and begin removing foreign materials, bacteria, and damaged tissue. Other white blood cells and macrophages also begin to arrive. This point signals the initiation of the inflammatory phase. Neutrophil numbers peak after 1−2 days and, in the absence of infection or further damage, rapidly decline. Macrophages continue to increase until day 5, their numbers then decrease slowly as healing proceeds. A significant population of macrophages will still be present for the proliferation phase. The inflammatory phase must be temporary. If there is an infection or other inflammatory process, this retards wound healing(9, 14, 15).
Once the inflammatory phase has produced a cleaned wound area proliferation begins. This is the ‘repair’ phase of wound healing as new types of cells move in to start the processes of granulation and epithelialization. Granulation fills the base of the wound bed with a nutrient and oxygen rich environment for epithelialization (the migration of epithelial cells over the wound surface) to take place(9, 15).
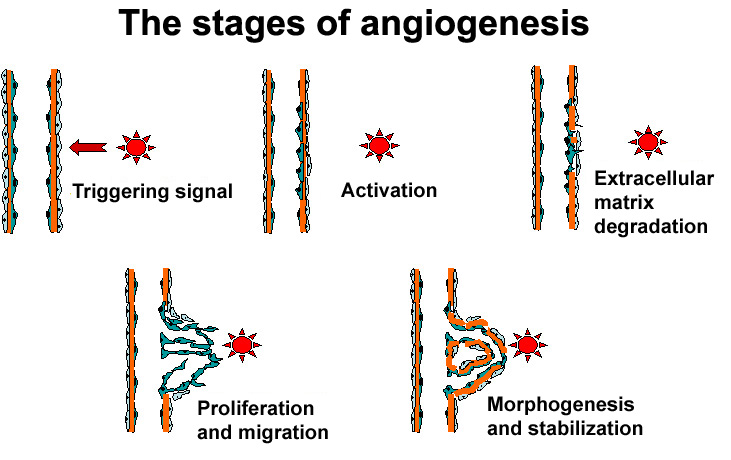
Granulation: Initially, ECM is deposited in an unorganised way. While the wound heals this matrix is continually degraded by proteolysis and resynthesized as needed. For example, for blood vessels to grow into the area (angiogenesis), capillary endothelial cells detach and, using proteases to dissect a pathway, migrate into the haphazard matrix. These capillary sprouts can grow up to a few millimeters per day and are what gives the granulating wound bed its red, ‘granular’ appearance(9, 14). As the granulation phase progresses, myofibroblasts grow across the wound, anchoring to the edges and start to contract, pulling the edges in and decreasing the wound area(14).

Epithelialization: At the edges of the wound keratinocytes proliferate and produce daughter cells to migrate into the wound bed. Proteolytic action is required to dissect a pathway for keratinocyte migration, the same as for angiogenesis. If a scab is present the keratinocytes have to burrow underneath the scab and phagocytose and digest any debris they may encounter, not just the ECM. But in a moist environment, without a scab, the migration is easier and healing is accelerated. This discovery led to development of the concept of moist wound healing(14). While this new layer of cells is delicate, most modern wound dressings do not remove them when the dressing is changed. However, if a dressing is allowed to dry out or adhere to the wound, traumatic removal of the dressing may harm the delicate new epithelial layer(9).
The process of re-epithelialization is complete when keratinocytes migrating from the wound margins reach each other and contact inhibition induces a cessation of migration. Because keratinocytes are located around adnexal structures, if the wound is superficial and these structures are intact the migration can occur from these sites. It is common to find ‘islands of epithelium’ in a large superficial wound. For full thickness wounds of the same size all of the migration must start at the wound edges, therefore re-epithelialization is slower(14).
Now that the wound is closed over and the immediate repairs to the skin are complete, the body has time (up to 2 years actually) to remodel the tissue. Proteolysis continues in this phase, degrading the existing ECM and resynthesizing and cross-linking the new matrix to achieve greater wound strength(9, 14). However, even after months of remodelling, the tensile strength of the repaired skin will not achieve more than 80% of the strength of non-wounded skin(14, 15).
The resultant scar tissue is brittle and less elastic than normal skin. Hair follicles and sweat glands do not grow back. Scar tissue is unattractive but it achieves the need of the body to restore skin barrier function and prevent the ingress of bacteria(14).
| Physiologic change | Pathologic change | Clinical significance |
| Thinning of epidermis and dermis | Increased vulnerability to mechanical trauma, especially shearing and friction | Increased incidence of skin tears |
| Flattening of dermal papillae | Increased risk of blister formation | Increased susceptibility to infection |
| Slowdown in turnover rate of epidermis; decrease in ratio of proliferative-to-differentiated keratinocytes | Delayed cellular migration and proliferation. | Increased time to re-epithelialization. |
| Decreased wound contraction | Longer healing times after injury or surgery | |
| Decrease in elastin fibres | Loss of elasticity | Lax skin and wrinkling, with loss of self-esteem and/or depression |
| Decrease in vascularity and supporting structures in dermis | Fragile, easily broken blood vessels. | Skin easily bruised (senile purpura) |
| Decreased wound capillary growth | Increased risk of wound dehiscence | |
| Decrease in vascular plexus, blunted capillary loops | Loss of thermoregulatory ability | Hypothermia, heat stroke |
| Changes in and loss of collagen and elastin fibres | Decreased tensile strength, lower layers more susceptible to injury | Increased risk of pressure damage to elderly skin, decubitus ulcers |
| Delayed collagen remodeling | Longer healing times after injury or surgery | |
| Impaired immune response | Impaired inflammatory response | Impaired wound healing |
| Impaired delayed hypersensitivity reaction | Increased risk of severe injury from irritants | |
| Decreased production of cytokines | Impaired immune function | |
| Decrease in number of Langerhans cells | Increased susceptibility to photocarcinogenesis, false-negative delayed hypersensitivity tests | |
| Impaired neurologic responses | Reduced sensation | Increased risk of thermal or other accidental injury |
| Decreased skin thickness | Loss of cushioning and support | Increased risk of pressure damage, decubitus ulcers Increased susceptibility to skin tears, bruising |
| Decreased vitamin D precursor production | Osteoperosis and bone fractures | |
| Atrophy of sweat glands | Decreased sweating | Less ability to thrermoregulate, hypothermia Dry skin, xerosis |
| Reduced stratum corneum lipids | Decreased ability to retain water | Variable response to topical medications, altered sensitivity to irritants |
| Structural changes in stratum corneum | Altered barrier function | Variable response to topical medications, altered sensitivity to irritants |
| Reduced movement of water from dermis to epidermis | Reduced epidermal hydration | Dry skin, xerosis |
| Decrease in melanocytes | Loss of ability to tan, greater susceptibility to solar radiation | Cutaneous neoplasms |
| Greying hair | Loss of self-esteem |
Copied from Farage et al (2009)(16)
1. Flour, M. The pathophysiology of vulnerable skin. World Wide Wounds 2009 [cited 2011 October 18]; Available from: http://www.worldwidewounds.com/2009/September/Flour/vulnerable-skin-1.html.
2. McGrath, J.A. and J. Uitto, Anatomy and Organization of Human Skin, in Rook's Textbook of Dermatology, T. Burns, et al., Editors. 2010, Wiley-Blackwell: Oxford, UK. p. 3.1-3.53.
3. Watkins, P., Using emollients to restore and maintain skin integrity. Nursing Standard, 2008. 22(41): p. 51-57.
4. Bianchi, J. and J. Cameron, Assessment of skin integrity in the elderly 1. British Journal of Community Nursing, 2008. 13(3): p. S26.
5. Thomas, D.R. and N.M. Burkemper, Aging skin and wound healing. Clinics in Geriatric Medicine, 2013. 29(2): p. xi-xx.
6. Nazarko, L., Wound Care Series: Part one: Consequences of ageing and illness on skin. Nursing and Residential Care, 2005. 7(6): p. 255-257.
7. Benbow, M., Maintaining skin integrity and preventing pressure damage. Nursing and Residential Care, 2009. 11(9): p. 443.
8. McGrath, J.A. and J. Uitto, Chapter 3. Anatomy and Organization of Human Skin, in Rook's Textbook of Dermatology, T. Burns, et al., Editors. 2010, Wiley-Blackwell: Oxford, UK.
9. Strecker-McGraw, M.K., T.R. Jones, and D.G. Baer, Soft tissue wounds and principles of healing. Emergency medicine clinics of North America, 2007. 25(1): p. 1-22.
10. Elias, P.M., et al., Update on the structure and function of the skin barrier: Atopic Dermatitis as an exemplar of clinical implications, in Seminars in Cutaneous Medicine and Surgery, K.A. Arndt, P.E. LeBoit, and B.U. Wintroub, Editors. 2013, Frontline Medical Communications Inc.
11. Venus, M., J. Waterman, and I. McNab, Basic physiology of the skin. Surgery, 2010. 28(10): p. 469-472.
12. Eichenfield, L.F., et al., Understanding skin barrier differences: A demographic, cultural, and medical diversity viewpoint, in Seminars in Cutaneous Medicine and Surgery, K.A. Arndt, P.E. LeBoit, and B.U. Wintroub, Editors. 2013, Frontline Medical Communications Inc.
13. O'Toole, E.A. and J.E. Mellerio, Wound Healing, in Rook's Textbook of Dermatology, T. Burns, et al., Editors. 2010, Wiley-Blackwell: Oxford, UK. p. 14.1-14.27.
14. Moore, K., Cell Biology of Normal and Impaired Healing, in Microbiology of Wounds, S. Percival and K. Cutting, Editors. 2010, CRC Press: Boca Raton, Fla. p. 151-186.
15. Hermans, M.H.E. and T. Treadwell, An Introduction to Wounds, in Microbiology of Wounds, S. Percival and K. Cutting, Editors. 2010, CRC Press: Boca Raton, Fla. p. 83-134.
16. Farage, M.A., et al., Clinical implications of aging skin: cutaneous disorders in the elderly. American Journal Of Clinical Dermatology, 2009. 10(2): p. 73-86.
© 2015 Wound Care Resource All rights reserved | Web template modified from W3layouts