(download as a pdf - 9.2Mb)
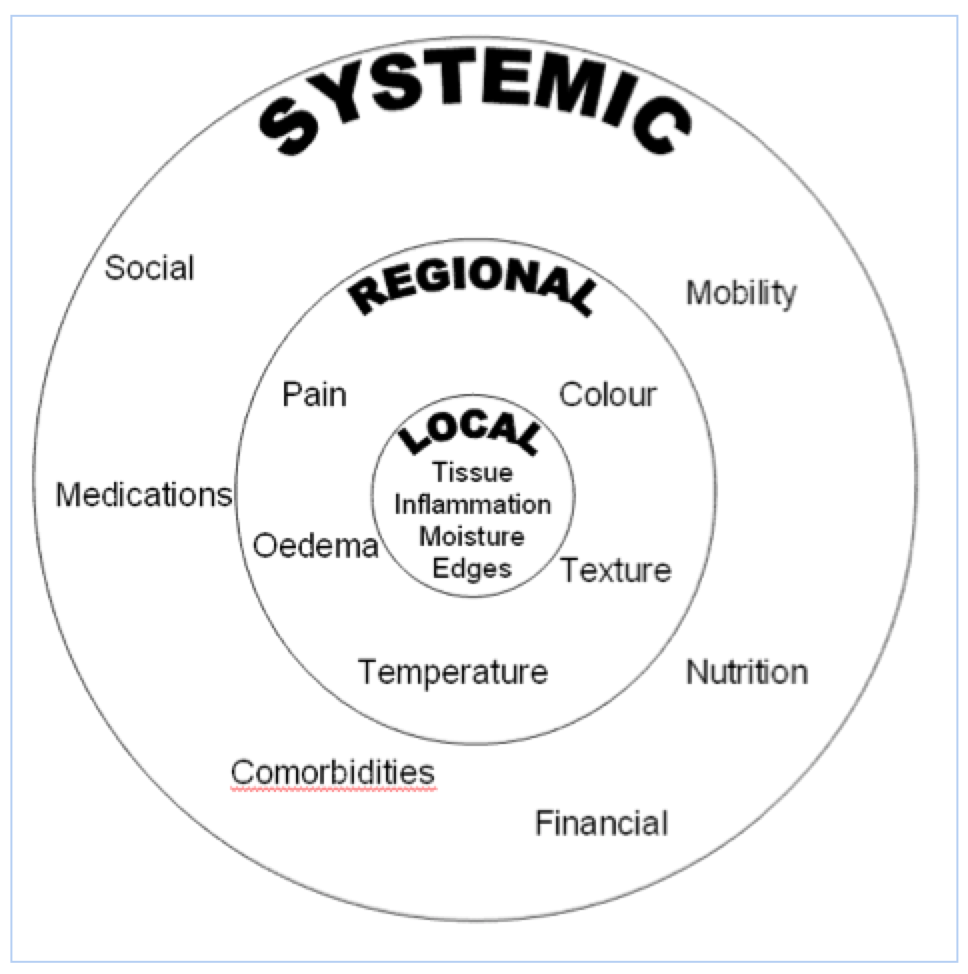
Years ago, cleverer people than I could see patterns emerging where if certain things were done there would be better wound healing outcomes. They pulled all these ideas together and wrapped mnemonics around them to make them easier to remember. Two mnemonics that work well together are HEIDI and TIME. These stand for History, Examination, Investigation, Diagnosis and Implementation (HEIDI) and Tissue, Inflammation, Moisture and Edges (TIME). To help remember these I have included a data collection tool you can use, see Appendix A. Now we will go into ALOT more detail about each of these.
It is important that your assessment considers the WHOLE patient, not just the HOLE in the patient(1). Start by considering what systemic factors might impact on wound healing or impact on your plan.
This is certainly NOT comprehensive, but to give you an idea of what some of these things might look like:
| Systemic | impact on | |
| ability to heal | plan | |
| Medical | Poor circulation – how will the nutrients get to the skin, how will waste/oedema be taken away? Poor oxygenation – how much oxygen is making it to the skin? Metabolic – what impact does diabetes have on wound healing? Auto-immune – for reasons not yet fully understood the body attacks it’s own organs, including the skin and supporting structures. | immune compromised - will not show typical signs of infection, do you watch for other signs or use a topical antimicrobial prophylactically? impaired sensation – can not feel if compression is too tight. |
| Surgical/Iatrogenic | Alteration to lymph system such as in lymph node removal for Cancer – can lead to oedema Previous scar tissue – such as from radiation or burns – structure is different to normal skin and slower to heal, can be the source of malignancy Gate changes – amputation will change gate, causing abnormal pressures in other areas of the foot, potential for further ulceration in those new areas. | working around surgical sites – applying a VAC around ex-fix pins managing exudate from a stoma or fistula |
| Nutrition | Not eating well – often related to age Vegetarian | expect delay – higher protein and caloric intake is required for wound healing |
| Social | Not mobile – pressure related tissue damage, poor calf muscle pump Poor housing or income – poor environmental controls can impact on healing Smoking – reduced oxygen to skin | on feet all day – off-loading? Venous return? cost - can not afford dressings |
| Medications | Corticosteroids Anti-inflammatories Anti-coagulents | Warfarin – would you debride? |
| Allergies | Adhesives Iodine Chlorhexadine | |
The information we get from the patient’s history gives us a list of items that may result in impaired healing or the potential for skin breakdown. We need to plan to mitigate the impact of as many as we can; ie education on quitting smoking, refer to specialist, or choosing more affordable dressings as well as any home support they might have or need.
This is where we start to get our hands on the patient. So, continuing on from above, we now need to assess regional symptoms that will need to be managed to improve wound healing.
| Regional | impact on | |
| ability to heal | plan | |
| Oedema | oedema makes it difficult for adequate distribution of nutrients to feed the skin | Can it be managed? |
| Pulses | indicates ability of nutrients to get to the area | Present? Not present? Do you need to collaborate? |
| Atrophy, no hair, thin shiney skin | Often associated with lack of pulses, may indicate poor arterial supply, possible claudication pain | Requires collaboration with Vascular as a minimum – do not debride |
| Haemosiderin staining, varicose veins, ankle flair, etc… | Often associated with oedema or an inverted champagne bottle appearance, may indicate poor venous return | Referral to Vein specialist, ABPI required, assessment for compression suitability |
| Dry cracked skin | Less resilient, increases risk of infection | non-soap cleaning, water intake?, humidity (air conditioning), moisturize |
| Charcot deformity | Changes to gate, potential for ulceration | Requires collaboration with podiatry as a minimum |
| Contractures | May be putting constant pressure onto certain areas or increasing build up of moisture in creases | Redistribute pressure as able. Manage moisture. Collaborate with occupational therapist and/or physiotherapist |
Again, not fully comprehensive but a start; see if you can come up with more!
NOW!! This is where we start to look at the wound (finally!).
We need to record the size and location of the wound. Serial size measurements need to be recorded as they indicate whether or not a wound is healing; one source recommends that a wound should be at least 30% smaller (surface area) by week 4(2) to be considered on a healing trajectory.
Measurement of the wound can be done in several ways:
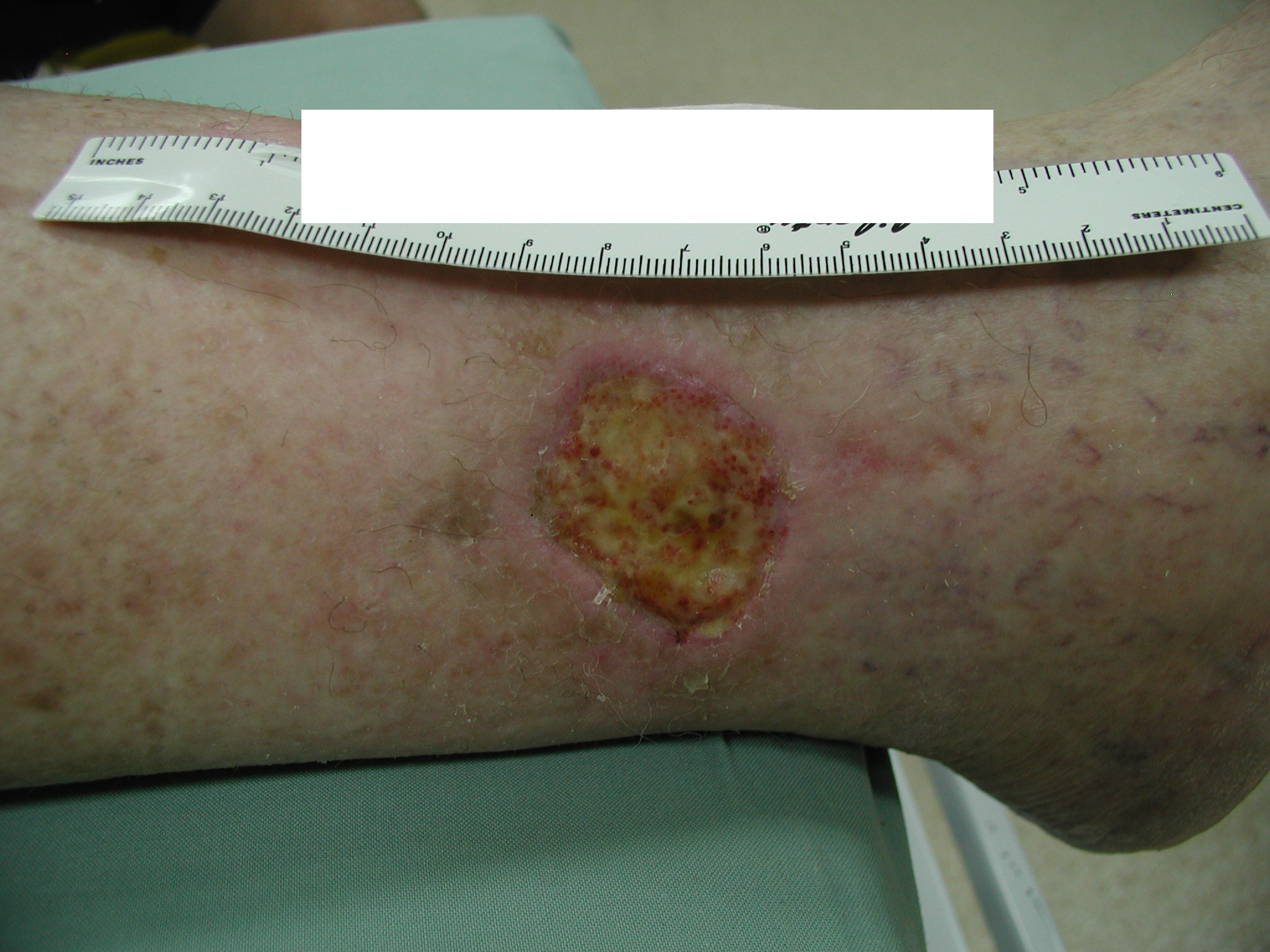
Prior to measuring the wound, clean the wound. If planning to debride, conduct measurements after debriding. Position the patient in a comfortable position keeping mind that positioning, body curvature, or tapering of the limbs will impact on the accuracy of the various techniques(3). Also ensure that all measurements are taken of the wound base, correct identification of wound margins has a large impact on wound size accuracy(4).
When using a ruler, measurements are taken of the greatest widest and the greatest length perpendicular to the greatest width(5). This is a quick method and works best with regularly shaped, small to medium sized wounds like the one in the photo on the right(3, 4). Multiply length x width to convert to an area measurement.
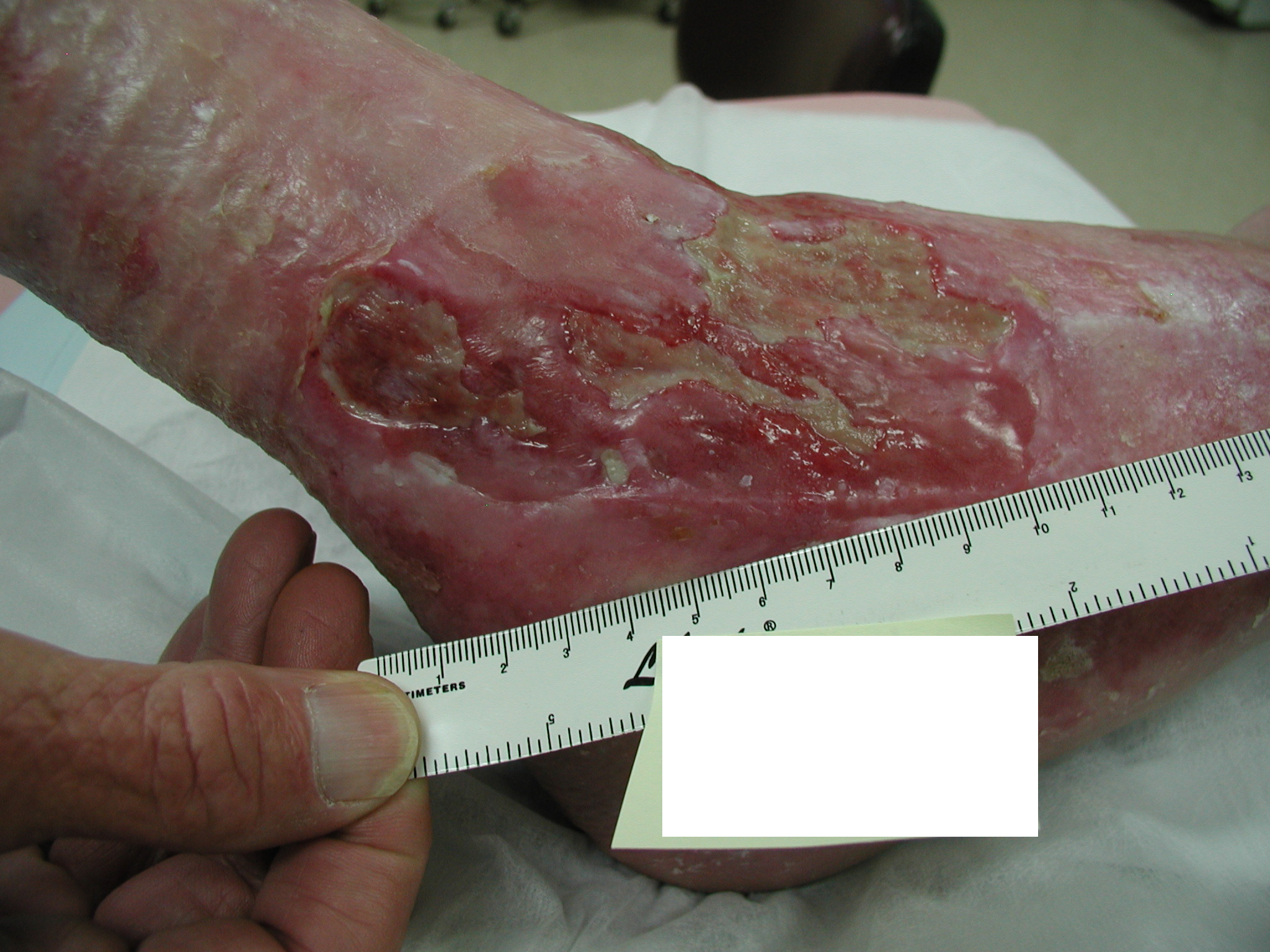
In irregularly shaped wounds, it is more accurate to trace the wound onto acetate and measure the area by placing the tracing on grid paper and adding up the number of squares contained within the margin of the outline of the wound. While this is considered more accurate for irregularly shaped wounds, error comes into play when trying to determine how to include partially covered squares(3, 4). Consider the wound on the right and ask yourself how would you go measuring this wound? Would you use a ruler or acetate?

Visitrak is a device that was created by Smith and Nephew for wound measurement. It had a sterile acetate sheet with disposable backing for tracing the wound onto. You then laid the tracing onto the device and re-traced it with the device’s stylus. While this was very good for infection control and could quickly report height, width, area and circumference, there were potential for errors in accuracy related to retracing and also the size of the device limited the size of the wound you could use it on(4, 5).
There is software available for purchase such as MOWA, which allows the user to take a photograph with their camera, trace the wound edges in the photo, and it produces wound dimensions and recommends tissue type based on colour. There are also free programs such as ImageJ, which can be loaded onto a computer and also allows the user to trace the wound edges and it produces the dimensions. The accuracy of both of these methods will be affected by the curvature of the surface and the ability of the user to trace the wound edge accurately.

A number of specialized photographic devices are on the market today. The SilhouetteStar is a camera which uses laser technology to measure wound depth and digital planimetry (where the user traces the wound edge in the photo) to measure area. The manufacturer claims a high degree of accuracy and ease of use. You need to have a computer or tablet with the Silhouette software attached to the camera.
While the SilhouetteStar does have a method for calculating depth, in all other instances the user will need to do this manually. Also, even when using the SilhouetteStar, undermining is not detected and, again, will need to be measured manually with a sterile probe. There are a number of devices that can be used including cotton-tipped swabs and sterile plastic sticks with measurement graduations on them.

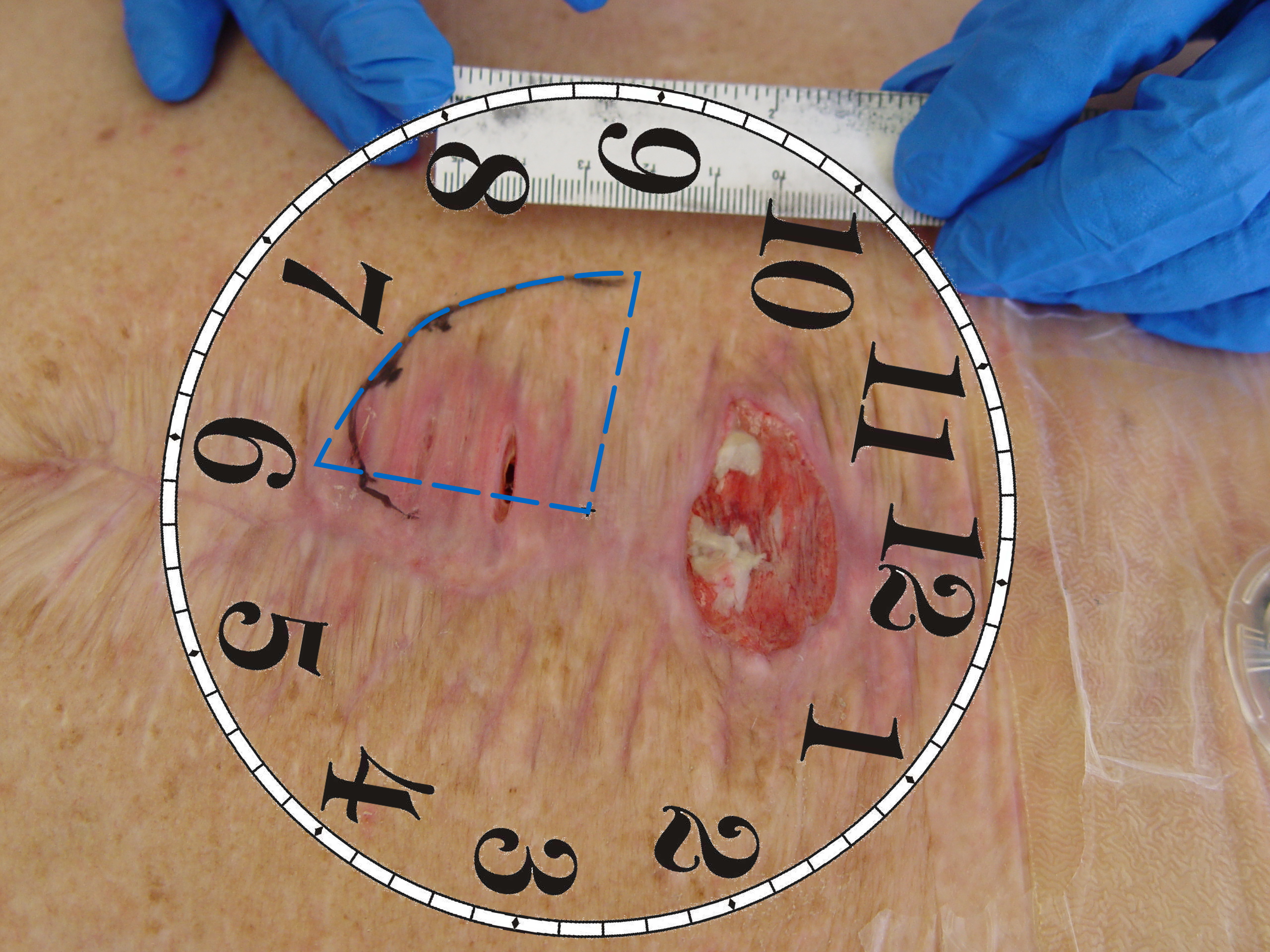
The depth gauge that was sold to accompany the Visitrak system was a thin plastic stick with measurement graduations and a foam tip. This was thin and flexible enough to measure most undermining/sinuses without fear of damaging surround tissue or leaving fibres behind. You will need to probe around the wound to find the greatest depth to record. Also, where there is a sinus or undermining, you will want to record the greatest depth and also the location at the wound edge. This is done by imagining a clock face over the wound with 12 o’clock being at the head. Therefore, in the picture on the right you would describe the undermining as being Xcm extending from 6 O’clock to 9 O’clock.
The location of the wound will also impact on determining a diagnosis and contribute to the plan(3). Below is a table showing locations and their likely correlations to wound type. However, this is just a guide and not a diagnosis.
| Site of wound & type of ulcer | |
| Site | Type of ulcer |
| Lower third of leg below knee | Venous ulcer |
| Bony prominences (e.g. heels, coccyx, sacrum, hips) | Pressure ulcer |
| Top of foot, bony prominences | Arterial ulcers |
| Ankles | Venous, arterial or pressure ulcer |
| Sole of foot & toes | Diabetic foot ulcers |
| Sun exposed areas | Skin cancers |
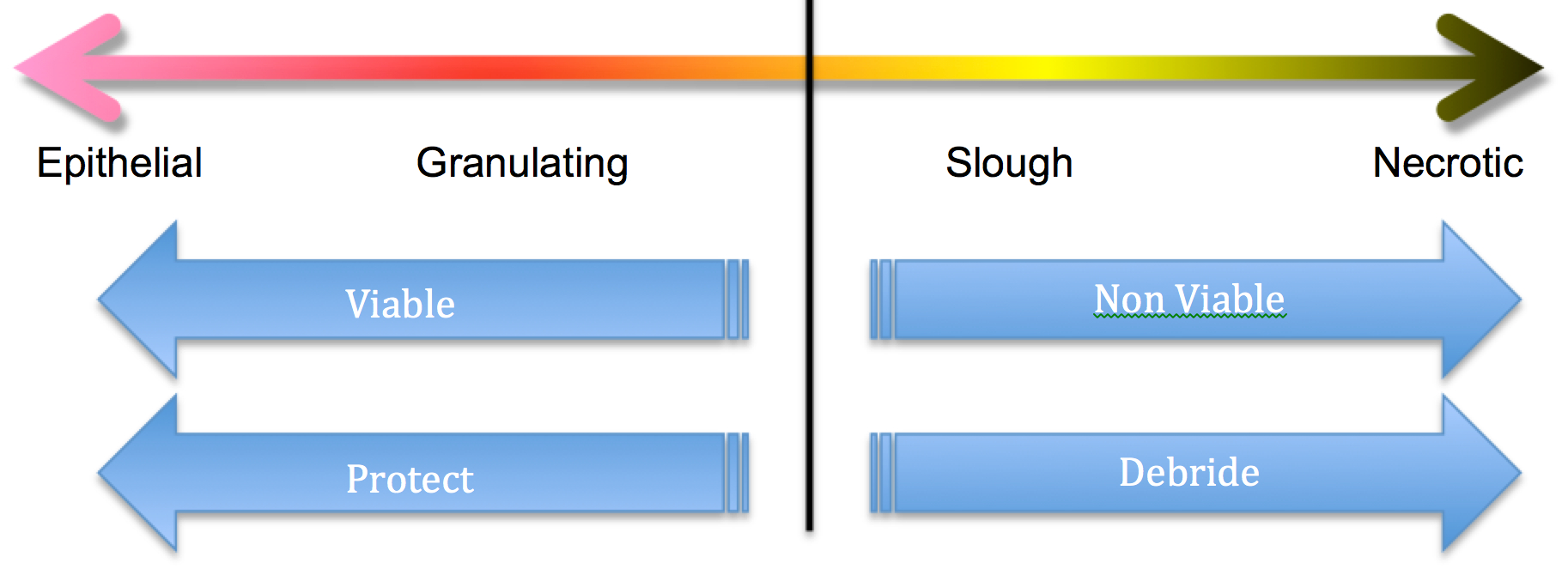
The image above is trying to convey that if the wound is greater than 50% granulating and epithelial, consider protection as your aim. If it falls to the slough and necrotic side then consider debridement.
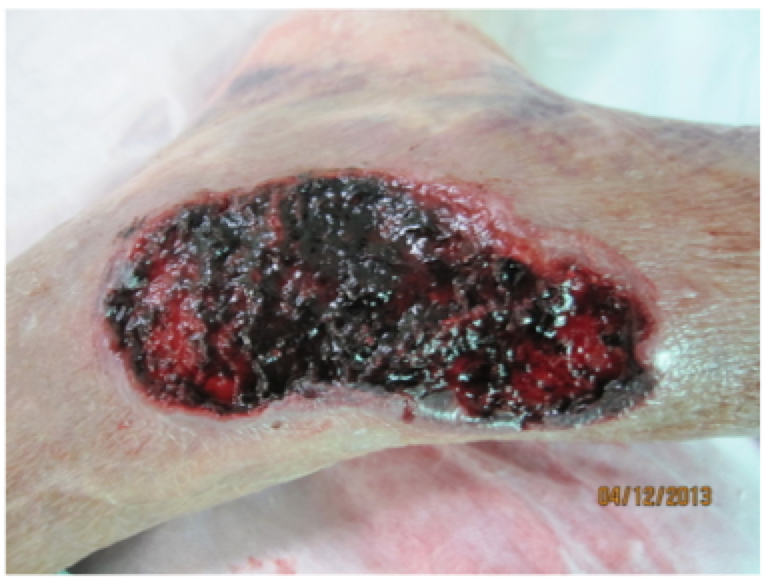 Tissue types tend to be described by colour. Pink is for epithelial, red for granulating, yellow for slough and black for eschar. Also consider other descriptors that may be helpful, these four alone do not always give you a good picture of what’s happening in the wound. The wound in the photo on the right could be described as 90% eschar and 10% granulation tissue. But that could also pertain to a crusty dry scab, and this is really wet; it’s actually coagulated blood from under a blood blister. So maybe rather than saying eschar it could be recorded as wet eschar or even clotted blood firmly adhered to the wound bed. Photos are a great way to record a wound, but even with a photo a good description can be very helpful.
Tissue types tend to be described by colour. Pink is for epithelial, red for granulating, yellow for slough and black for eschar. Also consider other descriptors that may be helpful, these four alone do not always give you a good picture of what’s happening in the wound. The wound in the photo on the right could be described as 90% eschar and 10% granulation tissue. But that could also pertain to a crusty dry scab, and this is really wet; it’s actually coagulated blood from under a blood blister. So maybe rather than saying eschar it could be recorded as wet eschar or even clotted blood firmly adhered to the wound bed. Photos are a great way to record a wound, but even with a photo a good description can be very helpful.
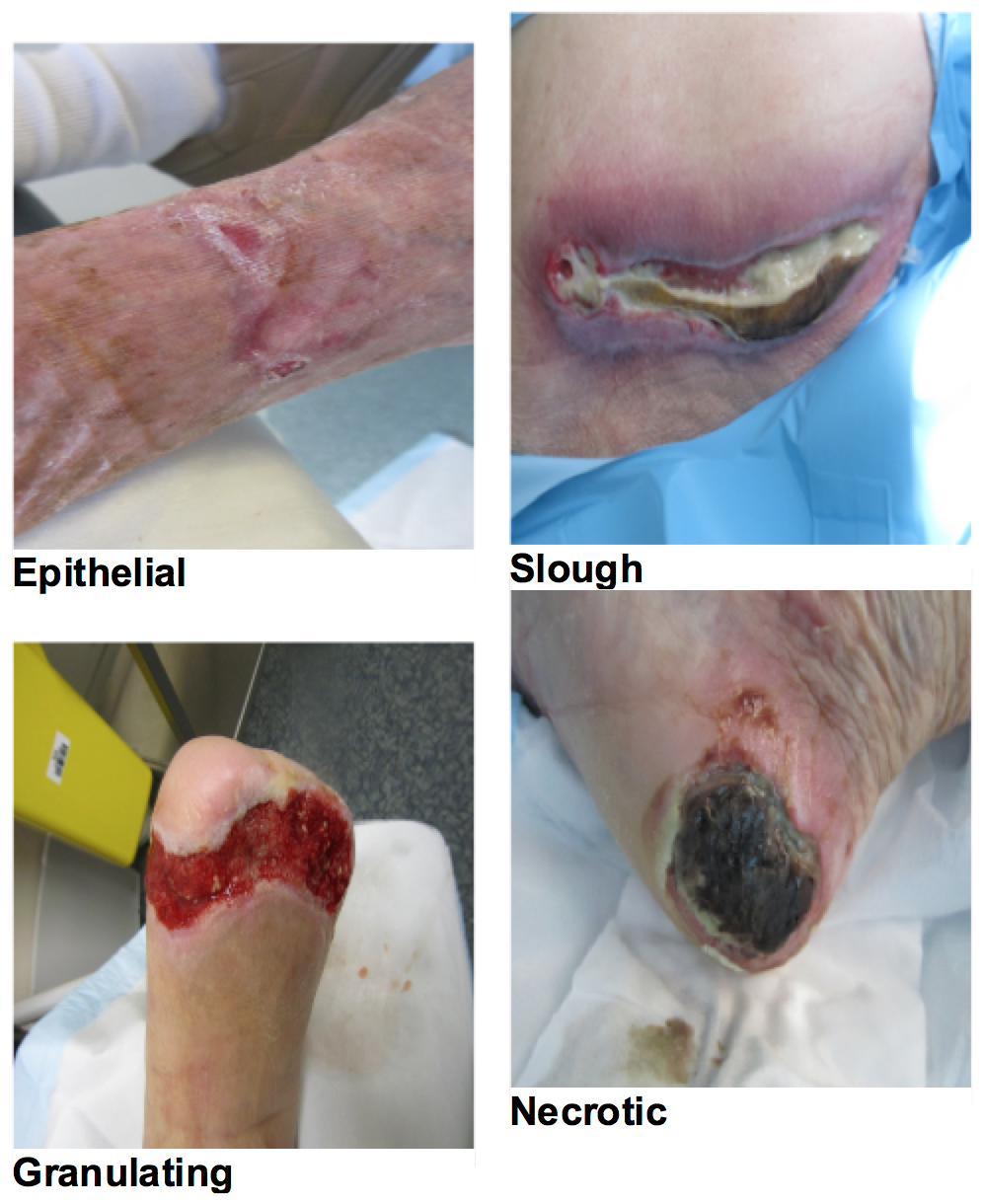 The epithelial and granulating tissue types are considered viable. These are alive and doing well. Slough and eschar are not viable, they do not have a blood supply to support healing and they are not alive. It is dead material and it needs to go. The presence of non-viable tissue and debris (foreign bodies/old dressing product/sutures)(6):
The epithelial and granulating tissue types are considered viable. These are alive and doing well. Slough and eschar are not viable, they do not have a blood supply to support healing and they are not alive. It is dead material and it needs to go. The presence of non-viable tissue and debris (foreign bodies/old dressing product/sutures)(6):
Debridement is the act of removing devitalized tissues from the wound bed. The act of debriding the wound bed not only improves the tissue in the wound bed but it also has a positive impact on inflammation (restores functional extracellular matrix proteins), moisture (reduces exudate) and edges (encourages migration) – the other three components of TIME. A number of things need to be considered before debriding(7):
If debridement is required you need to determine the best method. Most often as Nurses we make use of autolytic and mechanical debridement (the vigorous use of a surgisponge is considered mechanical debridement). But you need to be sure that debridement – whatever the chosen method – is safe.
Debridement is not always the preferred option for all wounds. In the case of the necrotic heel or toe in the patient with poor arterial flow. If the area is dry, leave it. It will eventually mummify and auto-amputate. Trying to moisten or remove the tissue is only likely to introduce new ways for microbial invasion that the body is not able to fight(8). Also, for the palliative patient where care is supportive, again if it is dry and not causing any pain, leave it(9). Fungating wounds are challenging in that they produce copious amounts of exudate and odour but are highly vascular. Attempts to debride may cause catastrophic bleeding, however leaving it causes further damage to the surrounding skin from maceration and stress to the patient from the odour(10). Cleaning with something like Prontosan helps to reduce the bioburden, which in turn reduces the exudate and the odour(11).
Debridement Methods
| Type | Mechanisms of action | Advantages | Disadvantages | Who/where |
| Autolytic | Uses the body’s own enzymes and moisture to rehydrate, soften and liquefy hard eschar and slough using occlusive or semi-occlusive dressings and/or antimicrobial products to create a balanced moist wound environment either by donating or absorbing moisture | Can be used for pre-debridement, when there is a small amount of non-viable tissue. Also suitable for wounds where other forms of debridement are inappropriate. Can be used for maintenance debridement. | The process is slow, increasing potential for infection and maceration. | Can be done by both generalist and specialist. |
| Biosurgical | Larvae of the green bottle fly are used to remove necrotic and devitalised tissue from the wound. Larvae are also able to ingest pathogenic organisms in the wound. | Highly selective and rapid. Costs are higher than autolytic debridement, but treatment is short once in place. | Not suitable for all patients or wounds | Can be applied by generalist or specialist practitioner with training. Closed bag method reduces skill level required and can be left for 4-5 days |
| Hydrosurgical | Removal of dead tissue using a high energy saline beam as a cutting implement | Short treatment time and selective. Capable of removing most if not all devitalised tissue from the wound bed. | Requires specialist equipment. There is potential for aerosol spread and it is associated with higher costs. | Must be carried out by a specialist practitioner with relevant training. Can be used in a variety of settings. |
| Mechanical | Traditional method involves using wet to dry gauze that dries and adheres to the top layer of the wound bed, which is ‘pulled’ away when the dressing is removed. | Newer methods are more selective, faster and relatively pain-free. | Non-selective and traditional methods are potentially harmful. Requires frequent dressing changes and can be very painful for the patient. | Can be done by both generalist and specialist. |
| Sharp | Removal of dead or devitalised tissue using a scalpel, scissors and/or forceps to just above the viable tissue level. This does not result in total debridement of all non-viable tissue and can be undertaken in conjunction with other therapies (eg autolysis). | Selective and quick. No analgesia is required normally. | Clinicians need to be able to distinguish tissue types and understand anatomy as the procedure carries the risk of damage to blood vessels, nerves and tendons. | Can be done at the patient’s bedside or in clinic by a skilled practitioner with specialist training. |
| Surgical | Excision or wider resection of non-viable tissue, including the removal of healthy tissue from the wound margins, until a healthy bleeding wound bed is achieved. | Selective and is best used on large areas where rapid removal is required. | It can be painful for the patient and anaesthetic is normally required. It can be associated with higher costs. | Must be performed in the operating theatre by a surgeon, podiatrist or specialist nurses following training. |
| Ultrasonic | Devices deliver ultrasound either in direct contact with the wound bed or via an atomised solution (mist). Most devices include a built-in irrigation system and are supplied with a variety of probes for different wound types. | Immediate and selective. It can be used for excisional debridement and/or maintenance debridement over several sessions. | Availability issues due to higher costs and requirement for specialist equipment. Requires longer set up and clean up time (involving sterilisation of hand pieces) than sharp debridement. | Must be carried out by competent practitioner with specialist training in a variety of settings. |
Table copied from Debridement Made Easy(12)
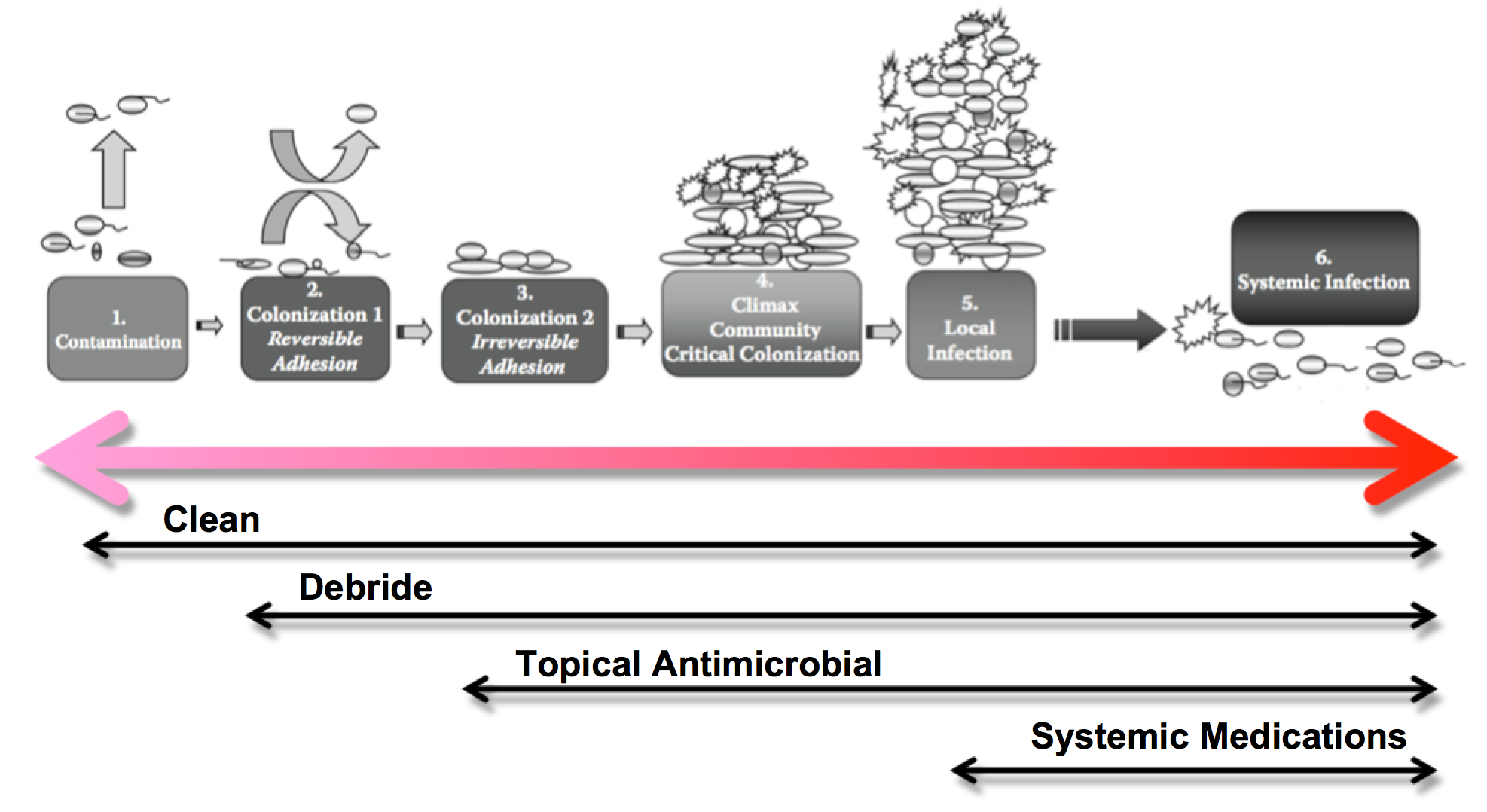
This image shows the progression from normal inflammation (where the wound is contaminated with opportunistic and transient pathogens) to systemic infection. Below each phase are the actions you can consider for each phase. They are cumulative: so for a wound that appears critically colonized you would want to clean, debride and use a topical antimicrobial.
Infection is the outcome of the dynamic interactions that take place between a host and a potential pathogen where the host defence strategies are successfully evaded and there is a negative impact on the host(13). Complex interactions leading to infection are not yet completely understood but have been grouped into 3 broad areas:
Contamination
Colonisation
Infection
The image shown above, the infection continuum arrow, is a modified version of the one in “The Microbiology of Wounds” by Percival & Dowd (14). They expand this spread from colonization to infection into 6 stages:
Stages of microbial invasion
| Contamination or Transient Stage | This is where bacteria are entering an area and assessing its suitability for colonization. They may have come from surrounding areas on the patient (endogenous) or from the environment or healthcare worker (exogenous). These bacteria are still in a free floating or ‘planktonic’ stage and are vulnerable to eradication. |
| Colonization Stage 1—Reversible Adhesion | If the bacteria decide that this is a suitable location to grow it will start to attach itself to the host cells using whatever adhesion options it has. This is called reversible adhesion because it is still possible to remove these bacteria with low levels of sheer force (eg saline rinses) and they are also still very susceptible to antimicrobial agents and host defenses. As time goes on the adhesion forces become stronger and the bacteria start to produce extracellular substances to make the area more suitable, thus starting the development of the biofilm. Early colonizing bacteria are typically Staphylococcus and beta hemolytic Streptococci. |
| Colonization Stage 2—Irreversible Adhesion | At this point the biofilm is now starting to alter the conditions in the wound, making it more difficult for the host defenses and antibiotics to get to the bacteria hiding in it. It is still possible to remove but with a greater amount of force required. This new biofilm encourages other bacteria to join the community. As the community grows and numbers of bacteria increase micro-colonies form and areas of the biofilm become hypoxic, thus creating a suitable environment for anaerobic bacteria as well. |
| Critical Colonization Stage—Climax Community or “Biofilm” | From a clinical perspective, this is where the wound is sufficiently colonized to exert it’s effects on the wound and prevent healing. From a microbiological point of view it is the critical mass of bacteria that has formed a viable colony but has not yet obtained high enough numbers to invade surrounding tissues (cause infection). There is no set number as different combinations of pathogens and host defenses mean this threshold will vary. |
| Local Infection Stage | Once the colony is mature, growth will continue exponentially and bacteria will be able to use this safe colony as a staging area to invade local tissues. A local wound infection will present clinically with redness (erythema), excessive pain, swelling, heat generation, wound breakdown and increased local temperature. Other more subtle clinical signs of infection have included alteration in exudate, friable granulation tissue that bleeds easily, malodor, and discolored granulation tissue. Reported granulation tissue discolouration has included yellow, green, or blue when bacteria such as Pseudomonas aeruginosa, Streptococci, and Bacteroides fragilis have been cultured. |
| Systemic Infection Stage | If the biofilm is not disturbed with appropriate wound bed preparation techniques, appropriate dressing management and the use of topical antimicrobials the colony will continue to produce invading bacteria which can lead to bacteria getting into the bloodstream (bacteremia) leading to sepsis or septicemia (multiplication of bacteria in the blood and toxin production), potential organ failure, and in extreme cases, death. |
Cleaning, debriding and appropriate use of antimicrobial dressings all help to reduce bioburden and reduce the establishment of a biofilm. Researchers are still unclear how long it takes a biofilm to form. There are many variables including strain of bacteria and it’s adhesion methods, host defenses and type of material being adhered to (ie skin vs implant). There are in-vitro studies (controlled environment, in a lab/not on a live subject) that indicate Staphylococcus aureus can create a biofilm within 2-3 hours and other studies on pig models that have established biofilms within 48 hours(15, 16). The theories behind why these bacteria form biofilms are also varied(17). Biofilms may be created for protection from host defenses, colonization of a nutrient-rich area and/or utilization of the cooperative benefits of a community(17).
The biofilm is known to enhance communal protection from phagocytosis by polymorphonuclear leukocytes (PMNs). This means that once the hardier species have started to establish a biofilm the more sensitive species will have a safe place to attach and grow. It may even make these sensitive species appear to be ‘resistant’. Once the oxygen starts to be depleted the anaerobes can also join the community. This synergy between aerobic and anaerobic bacteria has been documented to increase the severity of an infection(18, 19).
The site the bacteria choose to colonize may be nutrient rich, but the bacteria still need to be able to unlock those nutrients including glycoproteins, sugars, and proteins. In order to do this numerous enzymes are required, ranging from proteases to glycosidases. Having a community of bacteria with a range of different enzymatic properties means that more nutrients can be ‘unlocked’ which would benefit the community as a whole(14).
By creating communities where different bacteria inhabit niche micro-habitats best suited for their survival, and where the bacteria cooperate to meet their collective metabolic requirements, the biofilm is more likely to succeed. If we do not remove the biofilm, then the wound is less likely to heal(14, 20).
Identifying the difference between inflammation and infection can be tricky. Generally speaking, erythema, warmth, exudate and pain can be associated with both. In a person who is immunocompromised or has a reduced immune response for another reason (such as in the feet of people with diabetes) there may be no local sign that infection is present; the first signs could be rigors or pain at regional lymph nodes(21, 22). Chronic and acute wounds are also assessed differently because in acute wounds we have the window of the first 48 hours where we have an expectation of what the ‘normal’ wound healing will look like. We expect that for the acute wound, after 48 hours there should be a reduction in erythema, warmth, exudate and pain and if these do not reduce, or if they get worse, there may be a local infection.
For chronic wounds Sibbald et al(23) recommend two mnemonics to help remember what to look for and to also differentiate between superficial and deep bacterial burden. The difference being that superficial bacterial burden may respond to topical antimicrobials whereas deep infections usually require the use of systemic medications.
| For superficial infection, think of NERDS | For deep infection, think of STONES |
| Nonhealing wounds Exudative wounds Red and bleeding granulation tissue (friable) Debris (yellow or black necrotic tissue) Smell or unpleasant odour from the wound | Size is bigger Temperature increased Os [probe to or exposed bone] New or satellite areas of breakdown Exudate, erythema, edema Smell |
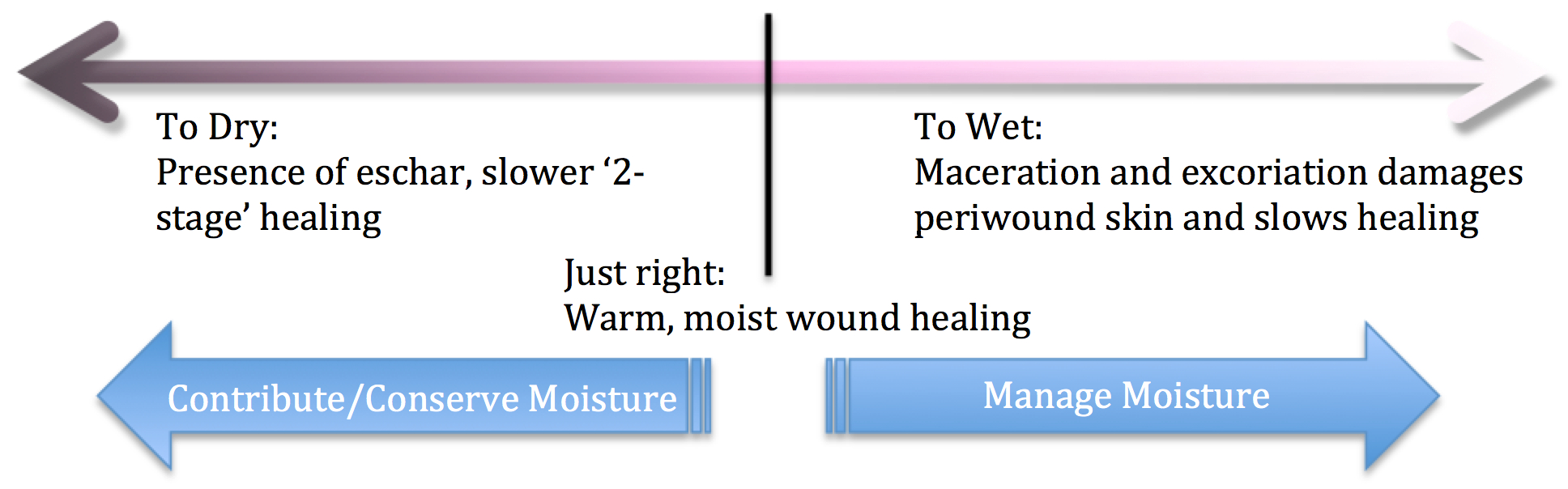

An imbalance in moisture results in:
The aim is to restore epithelial cell migration through the management of exudate and its underlying causes(24). At the edges of the wound keratinocytes proliferate and produce daughter cells to migrate into the wound bed. If a scab is present proteases have to break down and clear a path for the keratinocytes to burrow underneath the scab. But in a moist environment, without a scab, the migration is easier and healing is accelerated. This discovery led to development of the concept of moist wound healing(25). While this new layer of cells is delicate, most modern wound dressings do not remove them when the dressing is changed. However, if a dressing is allowed to dry out or adhere to the wound, traumatic removal of the dressing may harm the delicate new epithelial layer(26).
Moisture in a wound can be modified directly or indirectly(27):
General observations about dressings:

Wound edges that are:
Also, the edges can give us information to help diagnose the type of wound. Below is a table showing edge characteristics and their likely correlations to wound type. However, this is just a guide and not a diagnosis by itself.
Wound edge characteristics
| Edges | Type of wound |
| Sloping | Venous ulcer |
| Punched out | Arterial ulcer |
| Rolled | Basal cell carcinoma |
| Raised | Squamous cell carcinoma |
| Undermining | Pressure ulcer |
| Calloused | Diabetic foot ulcer |
| Purple | Vasculitic |
The other thing to remember is that these edges, and the periwound skin, need to be protected during the course of the wound treatment. Things like excess moisture and increased bioburden levels can affect the periwound skin. The use of adhesives can cause damage from their frequent removal or may trigger an allergic response. Products used to debride necrotic tissue can be just as ready to break down the healthy tissue so care must be taken.
The use of moisturizers/emollients and an adequate intake of water, daily, help to give the skin resilience(28). Simple periwound skin protection can be obtained by using barrier wipes, creams or sprays(11). In some cases, exudate management bags, hydrocolloid dressings and pastes need to be used to ensure adequate protection.
To try and simplify all of this information, a chart that plots tissue type, exudate levels and the presences/absence of infection can be used to help summarize options. But remember, every person is different, every wound is different, so use this as a guide only. And if a wound does not show improvement within 2-4 weeks of optimum care, do a full patient re-assessment and consider further collaboration with others.
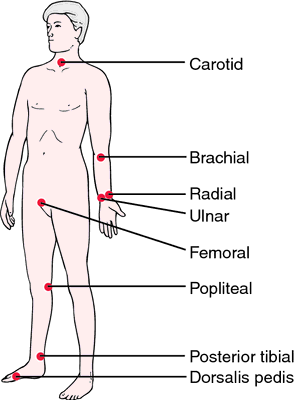
Do we have enough information to understand the underlying problem (diagnosis) and create a comprehensive management plan, or do we need more? Examples of investigations we can do at the beside are things like checking for the presence of pulses and sensation. Get into the practice (on healthy patients) of finding brachial, dorsalis pedis, posterior tibial and popliteal pulses. We don’t tend to use these often so don’t get much practice. Also, having, and knowing how to use, a monofilament pen or tuning fork to determine sensation perception is very useful.
Other investigations undertaken by the doctors include pathology and medical imaging. You can always recommend investigations be done when discussing your patient with the doctor. For example, in a wound with bone on view, it is reasonable to recommend imaging for osteomyelitis. In legs with signs of arterial or venous disease, it is reasonable to recommend an ABPI. For the wound bed that is friable, has an odd or pearly texture, especially where the patient has a history of skin cancers, it is reasonable to recommend a biopsy. The doctor may or may not wish to include this in their plan, but at least you have brought attention to a potential problem. Remember to use your SBAR communication skills when making these recommendations.
We understand a diagnosis to be the identification of a disease or medical condition by examination of the signs, symptoms and any tests performed(29). Some diagnoses are obvious, like when the patient hits their arm on the rollator and gets a skin tear. This is a skin tear (with appropriate STAR category) secondary to trauma. We can usually put a diagnosis to the acute wounds and some chronic wounds like pressure injuries. However, other chronic wounds that are not healing with optimum treatment may either have no diagnosis, or the wrong one. Let’s have another look at the woman who hit her arm on the rollator. The wound is cleaned, edges re-apposed, and dressing with compression applied. After a few months the woman is seen again, she still has a small area that appears to heal, then gets a bit crusty and starts bleeding again. The area is slightly raised compared to the surrounding skin. Is the diagnosis of skin tear still appropriate? Or should there be further investigations to determine what is happening at the cellular level to stop this wound completely healing? This is where we need to collect as much information and discuss the need for further investigations and possibly referral with the treating team.
Some examples of wound diagnoses are:
The important thing to remember is that we need to know what it is to be able to create the most appropriate care plan. Failure to correctly diagnose a wound type may result in failed management and wasted resources. Interventions based on accurate diagnosis delivers benefits to patients, healthcare systems and society(30). Let’s look at a person with chest pain. We only address the symptom (pain) with pain relief and when they are no longer in pain we send them home. What’s going to happen? Is the pain going to come back? Get worse? This patient is likely to re-present to hospital time and time again with the same symptoms, or worse. The underlying condition (whatever is causing the infarct) is not likely to improve by itself and will generally follow a deteriorating course. So now let’s look at a person with an infected ulcer. It’s been there a while, the antibiotics reduce the redness and swelling and the wound appears to start healing, but it has done this before, and will do it again. Why? We have no diagnosis so we don’t know why. Because we have not treated the cause we have wasted time and resources and the person with the wound has to continue to live with it and bear the costs, everyday. What if the diagnosis of the wound is a squamous cell carcinoma or peripheral venous disease? We need a diagnosis to ensure the wound is adequately managed and all contributory factors are addressed(29, 31). As our society continues to age, and lifestyle diseases continue to increase, the problem of pressure injuries and diabetic ulcers is growing. These and other common types of chronic wounds will require accurate and concise diagnosis and appropriate treatment as part of holistic care(2).
Once you have collected your history, done your examination, completed any other investigations and determined the wound diagnosis, you will be ready to put together your comprehensive wound care plan. In all of these previous steps you will have identified risks to healing or other things that will impact on healing or your care plan. For example:
| Findings | Plan | |
| History |
|
|
| Examination |
|
|
| Investigation |
|
|
| Diagnosis | ?Venous Leg Ulcer | Discuss with the treating team the need for a diagnosis and plan for compression. |
Communication and collaboration in wound care is essential. Gottrup(32) refers to a study done in one hospital in Copenhagen where they found in the majority of cases that:
Why? Did they not have a diagnosis or were the people looking after these patients missing the necessary skills? Gottrup proposes that to remedy this and provide optimum care for the complex wound care patient, care needs to be delivered by teams, and not individuals.
Since 1970 we have known that a standardized method for measuring wound healing is needed(34). Regular assessment, documenting progress and assessing the effectiveness of treatment maximizes healing rates(35). For chronic wounds, those wounds which do not heal in a timely manner, the benefits of using a standardized assessment tool can be significant(36). There are many factors, systemic, regional, local and environmental, that can impair wound healing and increase the risk of an acute wound becoming a chronic wound(1). The systematic assessment and collection of data minimises this risk(37). Where assessments are not performed correctly there is the risk of delayed healing and the potential for serious complications associated with living with a wound for a prolonged period of time(37). Not only are there risks associated with reduced skin barrier function, such as infection, but there is often pain, social isolation, and poorer quality of life(38, 39). Delayed wound healing requires additional nursing and medical resources, higher costs of consumables in wound care, and potentially higher costs of hospital lengths of stay to treat complications(37). The ever-expanding market of dressing products only adds to worsen the situation when you combine a poor assessment with an inappropriate and expensive dressing selection(34). Conversely, where skilled clinicians use a standardized framework which clearly guides Nurses from assessment through to implementing and monitoring wound care plans that correctly address the factors impacting on wound healing, healing times are reduced, patient suffering is reduced and the overall economic burden is reduced(36-38). By addressing the systemic causes of wounds and impaired healing, such as referrals to vascular or dermatological specialists, there is also the potential for reducing the risk of future wounds occurring or reducing their duration(40, 41).
The ideal wound assessment tool (WAT) will lead clinicians from assessment and diagnosis through to setting clear healing objectives and wound care plans. It will be grounded in research and evidence, and fast to use for clinicians of all knowledge levels(42-45). AWMA has created a set of standards for wound management(46) in which they include recommendations for assessment, planning and documentation. Their recommendations are summarized in Table 1. The recommendations from the AWMA standards adhere to this ideal and can be grouped as initial assessment (patient history and systemic observations), optional assessment (regional observations and investigations relevant to wound location and aetiology), ongoing assessment (wound bed and local area) and care planning (management plan, collaboration, documentation and evaluation)(46). These recommendations are very broad and AWMA does not provide specifics on how this should be done.
These recommendations were the basis for a literature review investigating a number of wound assessment tools and how they compared to the AWMA standard. You can read this review in full on my website (http:// woundcareresource.com/downloads/litreview-wat.pdf). The review shows that no single tool encompassed all recommendations from the AWMA standards, however the GCUH WCP covered more items than the comparison tools. This was still less than half of AWMA’s total recommendations.
While all the review articles agreed that comprehensive wound assessment is needed, such a comprehensive tool as outlined by AWMA would not be practical, and would not be used by Nurses. This was reflected in the audit of the GCUH WCP where (on average) half of the items on the tool were not completed. Nurses mainly used the WCP only as a means to record what dressings were applied to the wound. Arguably, the greater number of assessment items means the greater the ability to detect variation(47) but also the more time consuming to administer and therefore more costly(48). The modern care environment is one where nurses find they have more responsibilities, less time and high staff turnover rates; all of which are barriers to ensuring consistent use of a WAT(38, 45, 49). However, it is important to remember that correct completion of the WAT showing evidence based decision making processes which are clear, consistent, and coherent will reduce the risk of poor practice and, subsequently, the risk of litigation(50).
| Initial Assessment | Ongoing Assessment | Optional Assessment when indicated | Care Planning |
| Reason for Presentation | Wound type/Aetiology | Risk assessments (falls, skin integrity) | Short and long term goals |
| Health History | Duration | Vascular assessment | Management Plan to optimize wound healing potential |
| Age | Location | Sensory assessment | Individual and carer preference, ability and willingness to participate |
| Previous wound history and outcome | Dimensions | Nutritional assessment | Evidence of inter-professional communication and care |
| Medication history | Wound bed characteristics (tissue type and foreign bodies) | Psychological assessment | Comprehensive and chronological documentation |
| Psychosocial implications resulting from wounding | Wound edges appearance | Medical imaging | Effectiveness |
| Nutritional status | Peri-wound appearance | Pathology | Increase awareness of healthy lifestyle choices |
| Sensitivities and allergies | Exudate | Promote activity and mobility activities | |
| Relevant diagnostics and investigations | Odour | ||
| Pain assessment | Inflammation/Infection | ||
| Vital signs | Wound Pain | ||
| Individual’s perceptions of wound healing goals |
Collection of information in the digital age has allowed us to access the data easier, to quickly determine trends in healing, or to use the information for research (for example, assessing indicators of potential venus leg ulcer recurrence(51)). The internet provides us with a wealth of wound management information, some excellent (Wounds International, World Wide Wounds) and some which are, well let’s say, not peer reviewed… Within our healthcare settings the majority of our WATs are still paper-based and we continue to measure with disposable rulers. However, the digital age is encroaching on wound care.
In my opinion, the most significant of these has been the introduction of the digital camera. Being able to quickly, easily and accurately record the progress of a wound through these images has been incredibly helpful, increasing objectivity in temporal assessment. Wound measurement is also a hotly debated topic as, over the years, each new technology has promised to become the gold standard for wound measurement (um, no, we still don’t have one). The problem being, it is quick and easy to measure a wound with a ruler or trace it on a ruled piece of acetate and count the squares. These tools are cheap and easy to have handy. 3D cameras and the software to run them, digital planimetry devices and their consumables, all have high costs and take time to learn. While studies have shown that the accuracy of these more expensive devices is greater than that of the standard ruler measurements(52), the question is – how accurate do you need the measurement to be? Inter-rater and intra-rater reliability has regularly been shown to be high for simple measurements such as those with a ruler. However the ruler measurement itself has been proven to consistently over-estimate the wound size(53). So does it matter if the size is consistently over-estimated in the clinical setting? It will still show a trend, which is what is required to assist in clinical decision-making. But now we come to the point in time where everyone has a camera, and the software that can be used to calculate the wound size is either very cheap (MOWA) or free (ImageJ). While some of the pitfalls of wound photography are mentioned in another module, I think that it is safe to say that with the accuracy and non-contact nature of taking the photo(4), the minimal expense required, the tech-savvy nature of nurses and speed of measurement, this type of wound measurement will increase in clinical use.
1. Harding, K., et al., Evolution or Revolution? Adapting to complexity in wound management. International Wound Journal, 2007. 4 Suppl. 2(2): p. 1-12.
2. Sibbald, R.G., et al., Special considerations in wound bed preparation 2011: an update. World Council of Enterostomal Therapists Journal, 2012. 32(2): p. 10-30.
3. Grey, J.E., S. Enoch, and K. Harding, ABC of Wound Healing: Wound Assessment. British Medical Journal, 2006. 332: p. 285-288.
4. Chang, A.C., B. Dearman, and J.E. Greenwood, A comparison of wound area measurement techniques: Visitrack versus photography. ePlasty, 2011. 11: p. 158-166.
5. Bilgin, M. and Ü.Y. Güneş, A Comparison of 3 Wound Measurement Techniques. Journal of Wound, Ostomy & Continence Nursing, 2013. 40(6): p. 590-593.
6. Dowsett, C. and E. Ayello, TIME principles of chronic wound bed preparation and treatment. British Journal of Nursing, 2004. 13(15): p. S16.
7. O'Brien, M., Debridement: ethical, legal and practical considerations. Wound Care, 2003. March: p. 23-25.
8. unkown, Debridement in wound care. Wound Essentials, 2011. 6: p. 88-89.
9. Anderson, I., Debridement methods in wound care. Nursing Standard, 2006. 20(24): p. 65-72.
10. Benbow, M., Fungating malignant wounds and their management. Journal of Community Nursing, 2009. 23(11): p. 12.
11. Bradbury, S. and J. Fletcher, Prontosan made easy. Wounds International, 2011. 2(2): p. s25-s30.
12. Vowden, K. and P. Vowden, Debridement made easy. Wounds UK, 2011. 7(4): p. 1-4.
13. Cooper, R.A., Understanding Wound Infection, in European Wound Management Association (EWMA) Position Document: Identifiying Criteria for Wound Infection, S. Caine, et al., Editors. 2005, MEP Ltd: London.
14. Percival, S.L. and S.E. Dowd, Chapter 6. The Microbiology of Wounds, in Microbiology of Wounds, S. Percival and K. Cutting, Editors. 2010, CRC Press: Boca Raton, Fla.
15. Liesse Iyamba, J.M., et al., Study of the formation of a biofilm by clinical strains of Staphylococcus aureus. Biofouling, 2011. 27(8): p. 811-821.
16. Davis, S.C., et al., Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair And Regeneration, 2008. 16(1): p. 23-29.
17. Jefferson, K.K., What drives bacteria to produce a biofilm? FEMS Microbiology Letters, 2004. 236(2): p. 163-173.
18. Mastropaolo, M.D., et al., Synergy in polymicrobial infections in a mouse model of type 2 diabetes. Infection And Immunity, 2005. 73(9): p. 6055-6063.
19. Pollock, A.V., Wound infection: synergy between aerobic and anaerobic bacteria. Annals Of The Royal College Of Surgeons Of England, 1980. 62(3): p. 243-244.
20. Fazli, M., et al., Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. Journal Of Clinical Microbiology, 2009. 47(12): p. 4084-4089.
21. Richard, J.-L., J.-P. Lavigne, and A. Sotto, Diabetes and foot infection: more than double trouble. Diabetes/Metabolism Research and Reviews, 2012. 28: p. 46-53.
22. Edmonds, M., A.V.M. Foster, and P. Vowden, Wound bed preparation for diabetic foot ulcers, in European Wound Management Association (EWMA) Position Document: Wound Bed Preparation in Practice2004, MEP Ltd: London.
23. Sibbald, R.G., K. Woo, and E.A. Ayello, Increased bacterial burden and infection: the story of NERDS and STONES. Advances in Skin & Wound Care, 2006. 19(8): p. 447-461.
24. Dowsett, C., Moisture in wound healing: exudate management. British Journal of Community Nursing, 2011: p. S6-s12.
25. Moore, K., Cell Biology of Normal and Impaired Healing, in Microbiology of Wounds, S. Percival and K. Cutting, Editors. 2010, CRC Press: Boca Raton, Fla. p. 151-186.
26. Strecker-McGraw, M.K., T.R. Jones, and D.G. Baer, Soft tissue wounds and principles of healing. Emergency medicine clinics of North America, 2007. 25(1): p. 1-22.
27. Vowden, K. and P. Vowden. Wound Bed Preparation. 2002; Available from: http://www.worldwidewounds.com/2002/april/Vowden/Wound-Bed-Preparation.html.
28. Carville, K., Wound Care Manual. 5th ed2007, Osborn Park, WA: Silver Chain Nursing Association.
29. World Union of Wound Healing Societies (WUWHS), Principles of best practice: Diagnostics and wounds. A consensus document.2008, London: MEP Ltd.
30. Inernational consensus, Making the case for cost effective wound management. Wounds International, 2013.
31. Casey, G., Chronic wound healing: Leg ulcers. Kai Tiaki Nursing New Zealand, 2011. 17(11): p. 24-29.
32. Gottrup, F., A specialised wound care concept: The multi-disciplinary approach. The Chronical, 2009. 26(Spring 2009): p. 5-6.
33. Optimal Care of Chronic, Non-Healing, Lower Extremity Wounds: A Review of Clinical Evidence and Guidelines, 2013, Canadian Agency for Drugs and Technologies in Health.
34. Cooper, D.M., Human wound assessment: status report and implications for clinicians. AACN Clinical Issues in Critical Care Nursing, 1990. 1(3): p. 553-565.
35. Ferrell, B.A., The Sessing Scale for measurement of pressure ulcer healing. Advances In Wound Care: The Journal For Prevention And Healing, 1997. 10(5): p. 78-80.
36. Fletcher, J., Wound assessment and the TIME framework. British Journal of Nursing, 2007. 16(8): p. 462-4.
37. Greatrex-White, S. and H. Moxey, Wound assessment tools and nurses' needs: an evaluation study. International Wound Journal, 2013.
38. Cook, L., Wound assessment: exploring competency and current practice. British Journal of Community Nursing, 2011: p. S34-40.
39. Moffatt, C., et al., Psychosocial factors and delayed healing, in Position Document: Hard-to-heal wounds: a holistic approach, European Wound Management Association, Editor 2008, MEP Ltd: London. p. 10-14.
40. Martin, F. and A. Duffy, Assessing and managing venous leg ulcers in the community: a review. British Journal of Community Nursing, 2011: p. S6-s14.
41. Muir, C. and L. Watret, Managing wounds using a structured assessment tool. Journal of Community Nursing, 2006. 20(1): p. 10.
42. Kennedy, C. and D. Arundel, District nurses' knowledge and practice of wound assessment: 2. British Journal Of Nursing (Mark Allen Publishing), 1998. 7(8): p. 481-486.
43. Hess, C.T., The art of skin and wound care documentation. Advances in Skin & Wound Care, 2005. 18(1): p. 43-55.
44. Gray, D., et al., Using the wound healing continuum to identify treatment objectives, in Applied wound managment supplement. Part 2 Implementation, D. Gray, R. White, and P. Cooper, Editors. 2005, Wounds-UK: Aberdeen.
45. Best Practice Statement: Optimising wound care. Wounds UK, 2008.
46. Australian Wound Mangement Association, Standards for Wound Management. 2nd ed2010: Australian Wound Mangement Association. 31.
47. Matsui, Y., et al., Development of the DESIGN-R with an observational study: An absolute evaluation tool for monitoring pressure ulcer wound healing. Wound Repair & Regeneration, 2011. 19(3): p. 309-315.
48. Barber, S., A clinically relevant wound assessment method to monitor healing progression. Ostomy/Wound Management, 2008. 54(3): p. 42-49.
49. Maklebust, J., PUSH tool reality check: audience response... proceedings of the National Pressure Ulcer Advisory Panel, Fifth National Conference, "Monitoring Pressure Ulcer Healing: an Alternative to Reverse Staging". Advances in Wound Care, 1997. 10(5): p. 102-106.
50. Gray, D., et al., Applied wound management, in Applied wound management supplement, D. Gray, Editor 2004, Wounds-UK: Aberdeen.
51. Finlayson, K., H. Edwards, and M. Courtney, Relationships between preventive activities, psychosocial factors and recurrence of venous leg ulcers: a prospective study. Journal of Advanced Nursing, 2011. 67(10): p. 2180-2190.
52. Fette, A.M. A clinimetric analysis of wound measurement tools. World Wide Wounds, 2006.
53. Brown, D., Comparing different ulcer measurement techniques: a pilot study. Primary Intention, 2003. 11(3): p. 125-134.
54. The Australian Wound Management Association (AWMA) Pan Pacific Guideline Development Steering Committee, Pan Pacific Clinical Practice Guideline for the Prevention and Management of Pressure Injury.2012, Osborne Park, WA: Cambridge Media.
55. European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel, Treatment of pressure ulcers: Quick Reference Guide2009, Washington DC: National Pressure Ulcer Advisory Panel.
© 2015 Wound Care Resource All rights reserved | Web template modified from W3layouts