(download as a pdf - 3Mb)
Due to similar locations and presentations, moisture lesions are often misclassified as pressure ulcers(1). Training programs which help to teach us to differentiate between the two have been shown to be beneficial in producing more accurate diagnoses(2). The two aetiologies each have a unique treatment focus so we need to understand the differences between them. Differences are summarized in Appendix A.
IAD is inflammation of the skin of the perianal or genital areas, buttocks, or upper thighs due to prolonged contact with faeces, urine or sweat(3). Depending on the severity it may or may not be associated with loss of superficial skin layers and/or secondary infections(4). The presence of infection, often by opportunistic fungi, further increases morbidity(5).
In individuals with darker-toned skin IAD may present as areas of hypo- or hyper-pigmentation(5).
Reported prevalence rates vary greatly from 5% to almost 50% in the literature. The lower numbers are reported from nursing homes, mid range numbers are from hospitals (as a percentage of incontinent patients) and the highest rates are seen in acute geriatric care facilities(4, 6). One study in an intensive care unit (ICU) found that 36% of the patients in the study developed IAD within an average of 4 days. The patients in this study had faecal incontinence and diminished cognitive awareness; these characteristics were determined to be significant independent risk factors for IAD development(5).
As the population ages IAD is likely to become more of a problem. It is estimated that 31% of older women and 23% of older men experience urinary incontinence. 12% of older people also experience faecal incontinence(3).
Components contributing to IAD are(6):
| Impairments in tissue tolerance | This includes but is not limited to aging skin, reduced skin perfusion (as might be related to oedema secondary to congestive cardiac failure), and presence of previous scar tissue. |
| Problems of the perineal environment | Incontinence, excessive perspiration |
| Altered toileting ability from daily use of restraints | Cognitive or physical ability, wheelchair bound, restraints |
Prolonged exposure of the skin to moisture leads to maceration, which weakens the epidermis. Friction (such as that from pulling against bedding, clothing or incontinence aids during repositioning) will have a greater impact on this weakened skin allowing it to shear away(3). Another source of friction is that created by skin folds(4).
The protective barrier of the epidermis relies on it’s acidic nature. Ammonia (formed when urease-producing bacteria in urine or faeces split urea) is alkaline. When urine or faeces is in prolonged contact with the skin, the pH of the skin increases, reducing it’s ability to combat infection(3). In addition, faeces contain coliform bacteria and digestive enzymes that encourage greater skin irritation(4).
The main aim when treating IAD is to eliminate the cause - prolonged moisture on the skin. Through cleaning and the use of barrier protection we will also try to simulate the normal function of the skin until it has had time to repair itself.
Contain or divert the fluid. Absorbent pads are the mainstay of incontinence management and they are remarkably sophisticated in their absorbency capacity and also their ability to draw moisture away from the skin. However, it has been shown that even these products can actually increased tissue interface pressures when soaked, even when used in conjunction with pressure-reducing or relieving support surfaces(4). Therefore they should be changed often and in accordance with manufacturer’s instructions. They are also not able to draw away any solid matter that may be present in faecal incontinence. A study into the use of perianal pouches for faecal incontinence showed a reduced incidence of IAD and delayed onset compared to patients managed in adult containment briefs(4).
Clean the skin. Cleaning is also carried out regularly on all patients; incontinent patients tend to require more frequent perineal washes. Unfortunately, most skin cleansers have a pH of around 9, which is quite basic, and their frequent use in combination with course washcloths can increase stratum corneum swelling and alter lipid rigidity, stripping the skin of it’s natural barrier. Cleansing should involve a product whose pH range reflects the acid mantle of healthy skin (pH between 5.4 and 5.9)(3, 6, 7).
Provide a Barrier. A barrier is thought to assist in reducing the contact between the excess fluid and the skin. Depending on the barrier it may also help to reduce friction. There is some evidence that the use of a barrier can help to prevent and/or treat IAD(3). In a trial involving a washcloth that was impregnated with a barrier product only 8% of patients developed IAD as opposed to 27% of patients in the control group (n=141, P=0.003)(6). One popular barrier and cleanser washcloth combination has been shown to improve sin integrity in just a couple of days, not only in the groin but in other skin fold areas as well(7).
Moisturize the skin. Moisturising is known to be beneficial in protecting skin. It repairs the skin barrier, retains and increases water content, reduces transepidermal water vapour loss, and restores the lipid barrier by replacing the intracellular lipids(6). The presence of a moisturizer in the cleaning routine also appears to reduce IAD. In the ICU study, 10% of patients receiving the moisturizing cleanser developed IAD as opposed to 56% in the control group (n=45). This study also commented on the need for the cleansing routine to follow the patient to the ward post ICU, as complete healing had not been achieved in ICU(5).
There are many products on the market that provide barrier protection. There tend to be three base components, each of which have different strengths and weaknesses in relation to protection from irritation and ability to hydrate(2):
| Petrolatum | Soft white paraffin | Good protection against irritants and maceration, some skin hydration |
| Dimethicone | Cavilon | Varied protection against irritants, good skin hydration |
| Zinc oxide | Sudocrem, Amolin | Good protection against irritants, poor skin hydration |
A systematic review and meta-analysis by Beekman et al (2014)(8) looked at research that included urinary incontinence, faecal incontinence, double incontinence and moisture/microenvironment and the incidence and development of pressure injuries. It showed varying strengths to the relationship between the two but generally indicated that there is a clear association that if you have IAD you are at a greater risk of developing a pressure injury. One of the articles cited in the review created interesting food for thought. It stated that in nursing homes with high rates of pressure injuries there were high rates of faecal incontinence, but in nursing homes with low rates of pressure injuries there were still high rates of faecally incontinent residents. So does this mean if we are better at managing incontinence we will we be better at managing pressure injuries, or vice versa?
A pressure injury is a localized injury to the skin and/or underlying tissue, usually over a bony prominence, as a result of pressure or pressure in combination with shear. A number of contributing or confounding factors including, but not limited to, friction, microclimate, moisture, and nutrition are also associated with pressure ulcers; the significance of these factors has yet to be elucidated(1, 9, 10).
Reports from the UK indicate an 18% prevalence rate, USA reports 15%. These figures tend to be higher in palliative care/in home hospice, in patients with spinal cord injuries, and in critical care units. Associated with the incidence of PI are longer hospital lengths of stay and higher readmission rates. Recent European cost-models have indicated that the total costs of PIs may consume between 1% in the Netherlands and 4% in the United Kingdom of health care expenditure. They also have a substantial impact of quality of life for the patients and their carers(9).
Sixty thousand patients die each year from complications associated with PIs with an estimated cost of $11 billion per year to treat them (American data). PIs can delay discharge, expose the patient to potential serious infections, cause pain related to the ulcer and its treatment and is a poor indicator of mortality in an acute care setting(11). PIs are considered to be mostly preventable. Recently in Queensland a system has been introduced which penalizes hospitals for causing these injuries to patients. For a stage 3 or stage 4 PIs acquired in the hospital, the hospital will be fined $30k or $50k respectively.
Tissue loading is the defining characteristic of pressure injury formation. Pressure is increased in tissues that are positioned between a bony prominence and a support surface. Research has demonstrated that both magnitude of pressure and duration impact on pressure injury formation(12). Unrelieved pressure disrupts blood supply to the capillary network, impeding blood flow and depriving tissues of oxygen and nutrients. The most common sites for pressure injuries are the sacrum, heels, ischial tuberosities, greater trochanters, and lateral malleoli(13). Pressure is relieved and circulation restored by frequently turning and shifting weight distribution as well as with the use of dynamic surfaces that actively redistribute pressure on the body surfaces(12).
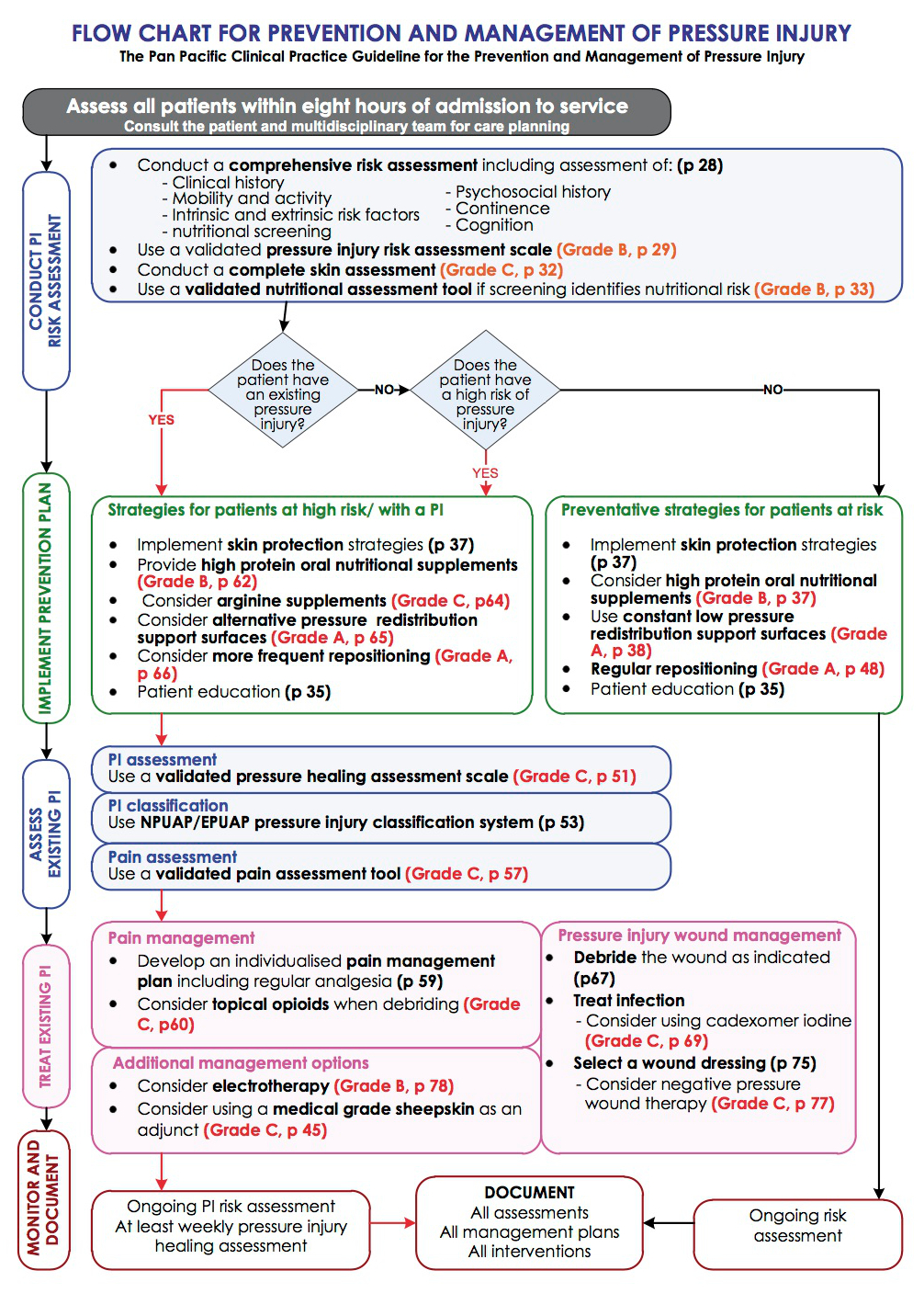
The recommended treatment of pressure injuries is multi-faceted, each part addressing the underlying cause or a contributing factor. The main cause of a pressure injury is pressure, so the main focus should also be on pressure removal/reduction. The flowhart of recommended treatment from the Pan Pacific Guidelines is in Appendix B.
Implement skin protection strategies. Skin protection is key in the prevention of PI’s. Intrinsic factors such as nutritional and hydration status (see “Refer to Dietician”) and external factors such as shear, friction and moisture, all need to be managed. The Pan Pacific Guidelines recommend(10):
Refer to Dietician. There is some research to support the use of additional nutritional support for the prevention and management of PIs where appropriate nutritional intake may be lacking. Oral nutrition support (ONS) includes supplements taken orally in addition to the patient’s regular diet. The most common forms of ONS considered for patients with PIs include(10):
Use appropriate support surfaces, appropriately. A support surface is a surface on which the patient is placed to manage pressure load, shear, friction and microclimate. This includes bed, trolley and operating table mattresses, integrated bed systems, and seat cushions. Support surfaces are designed to reduce interface pressure through increasing the body surface area or alternating the area of the body in contact with the support surface. It is important to note that the use of a support surface does not negate the repositioning requirements of a patient with a PI(10).
Make use of your available resources such as the PIP CNC, your Occupational Therapists and CERU when selecting an appropriate support surface for the severity of the PI and the patient’s general condition. Once the patient is using a support surface continue to monitor its effectiveness and consider trying something else if the patient’s skin condition is deteriorating and/or showing no signs of improvement. One example is that of the ROHO cushion where the patient puts the cushion behind their back instead of sitting on it.
Regular repositioning. Sustained pressure to areas of the body causes soft tissue injury. In normal circumstances, pain resulting from sustained injury prompts a person to change position. In patients who are unable to reposition themselves due to physical limitations, and in patients with reduced sensory perception and impaired ability to detect pain, failure to reposition is a significant risk factor for PIs. Regular repositioning is an essential component of PI prevention(10).
Tips for positioning in bed(10):
Tips for positioning in chairs/wheelchairs(10):
Correctly classify the PI. Use NPUAP/EPUAP pressure injury classification system(10, 14):
Category/Stage I: Non-blanchable redness of intact skin
Intact skin with non-blanchable erythema of a localized area usually over a bony prominence. Discoloration of the skin, warmth, edema, hardness or pain may also be present. Darkly pigmented skin may not have visible blanching. Further description: The area may be painful, firm, soft, warmer or cooler as compared to adjacent tissue. Category/Stage I may be difficult to detect in individuals with dark skin tones. May indicate “at risk” persons.
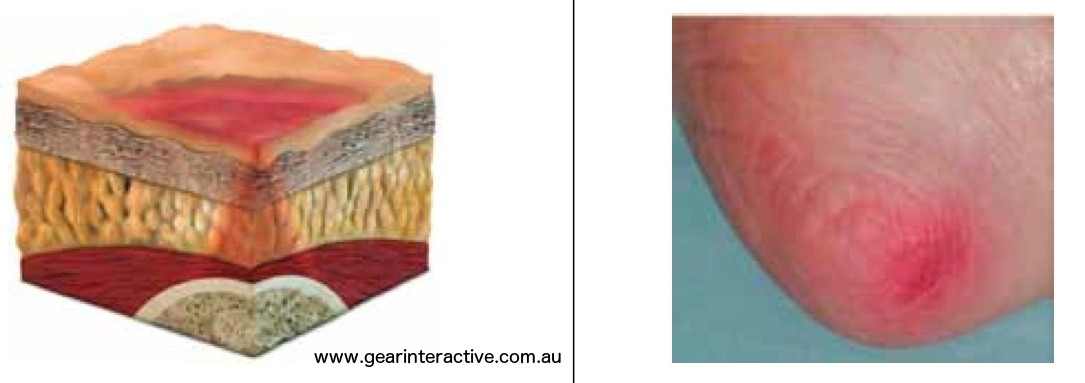
Category/Stage II: Partial thickness skin loss or blister
Partial thickness loss of dermis presenting as a shallow open ulcer with a red pink wound bed, without slough. May also present as an intact or open/ruptured serum- filled or sero-sanginous filled blister. Further description: Presents as a shiny or dry shallow ulcer without slough or bruising. This category/stage should not be used to describe skin tears, tape burns, incontinence associated dermatitis, maceration or excoriation.
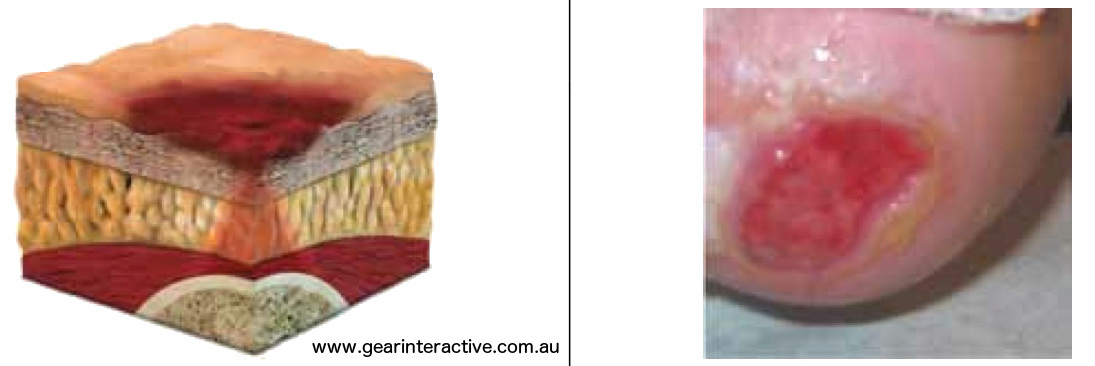
Category/Stage III: Full thickness skin loss (fat visible)
Full thickness tissue loss. Subcutaneous fat may be visible but bone, tendon or muscle are not exposed. Some slough may be present. May include undermining and tunneling.Further description: The depth of a Category/Stage III pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have (adipose) subcutaneous tissue and Category/Stage III ulcers can be shallow. In contrast, areas of significant adiposity can develop extremely deep Category/Stage III pressure ulcers. Bone/tendon is not visible or directly palpable.
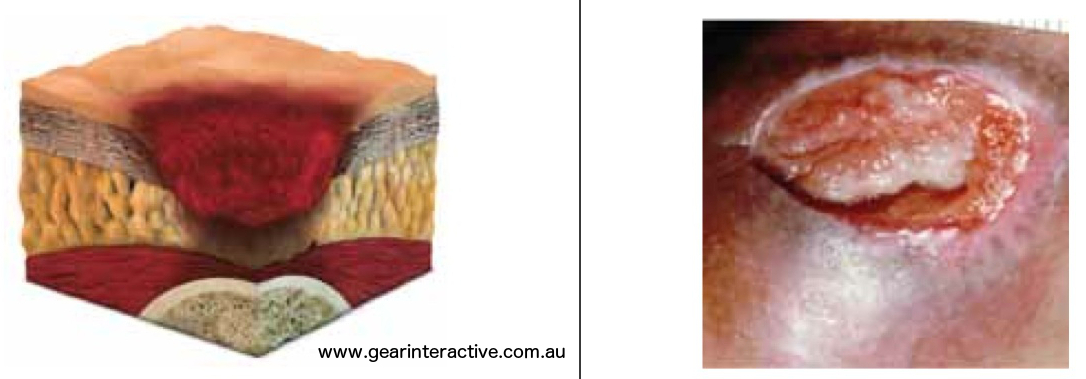
Category/Stage IV: Full thickness tissue loss (muscle/bone visible)
Full thickness tissue loss with exposed bone, tendon or muscle. Slough or eschar may be present. Often include undermining and tunneling. Further description: The depth of a Category/Stage IV pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have (adipose) subcutaneous tissue and these ulcers can be shallow. Category/Stage IV ulcers can extend into muscle and/or supporting structures (e.g., fascia, tendon or joint capsule) making osteomyelitis or osteitis likely to occur. Exposed bone/muscle is visible or directly palpable.
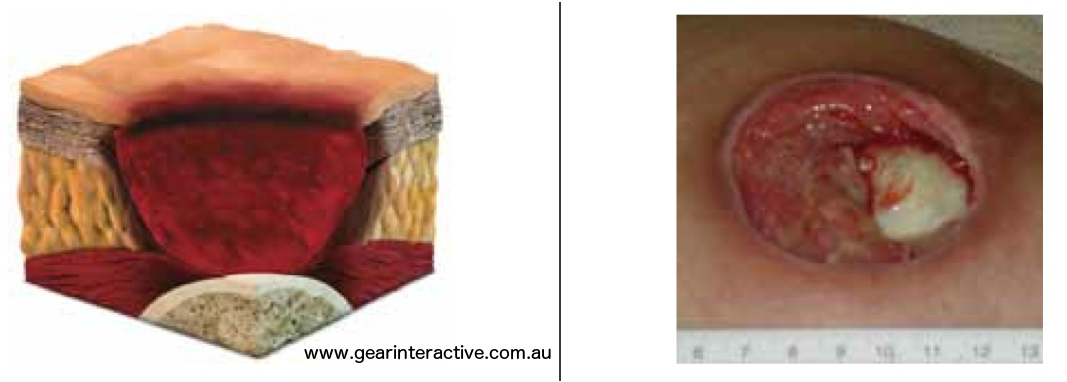
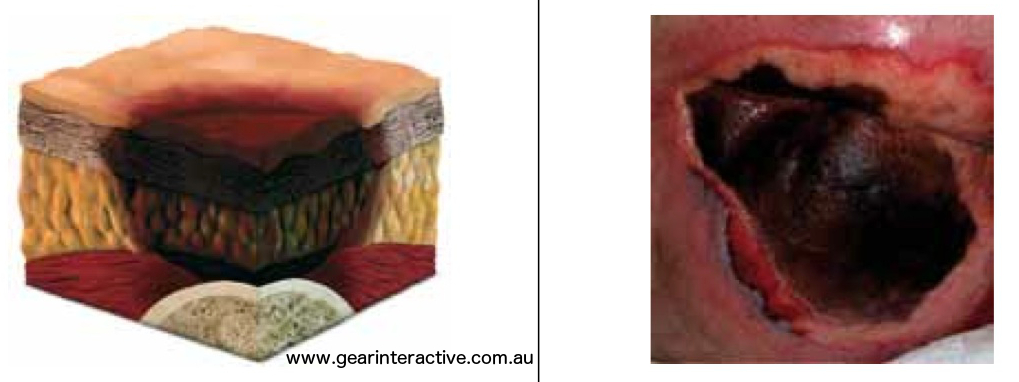
Unstageable/ Unclassified: Full thickness skin or tissue loss – depth unknown
Full thickness tissue loss in which actual depth of the ulcer is completely obscured by slough (yellow, tan, gray, green or brown) and/or eschar (tan, brown or black) in the wound bed. Further description: Until enough slough and/or eschar are removed to expose the base of the wound, the true depth cannot be determined; but it will be either a Category/Stage III or IV. Stable (dry, adherent, intact without erythema or fluctuance) eschar on the heels serves as “the body’s natural (biological) cover” and should not be removed.
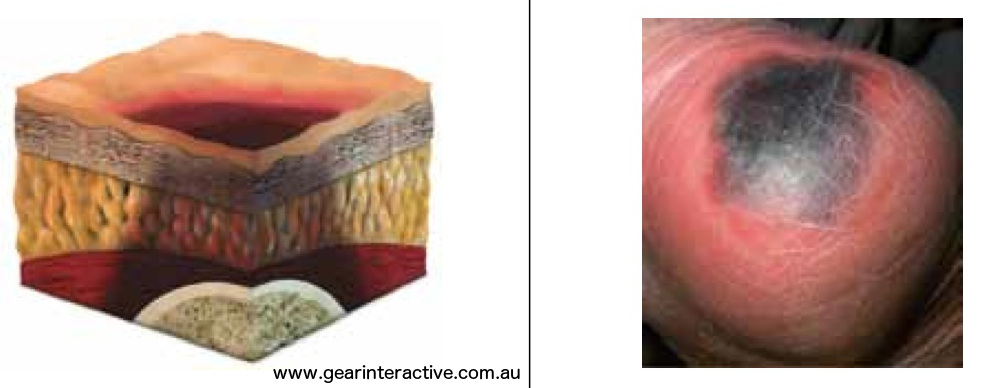
Suspected Deep Tissue Injury-depth unknown
Purple or maroon localized area of discolored intact skin or blood-filled blister due to damage of underlying soft tissue from pressure and/or shear. Further description: The area may be preceded by tissue that is painful, firm, mushy, boggy, warmer or cooler as compared to adjacent tissue. Deep tissue injury may be difficult to detect in individuals with dark skin tones. Evolution may include a thin blister over a dark wound bed. The wound may further evolve and become covered by thin eschar. Evolution may be rapid exposing additional layers of tissue even with treatment.
Debride the wound as indicated. Debridement is commonly performed on wounds to remove non-viable or infected tissue and debris in order to prepare the wound bed to receive therapeutic healing products (wound bed preparation). The aim is to maximise the healing process. Non-viable tissue can prolong the healing process by increasing inflammation, levels of bacteria and toxins, and inhibiting re-epithelialisation. Non-viable tissue generally presents as moist, yellow, green or grey in colour and over time becomes dry black or brown eschar. The most commonly used methods of debridement are(10):
Appendix C compares a list of debridement options.
When debridement is indicated, select the method of debridement with consideration to(10):
Surgical debridement is appropriate when there is an urgent need to remove non-viable tissue (e.g. advancing cellulitis, sepsis, pain, exudate or malodour). Conservative sharp wound debridement should only be performed by health professionals with appropriate training.
Treat infection. Antimicrobial therapy includes topical agents such as cadexomer iodine, silver, honey and other topical antimicrobials, as well as systemic antibiotics. All products should be used following comprehensive assessment and in accordance with the licensing authority endorsement and the manufacturers’ directions. Although all chronic wounds are contaminated or colonised, not all are infected. Signs of local infection in a pressure injury include(10):
Appendix D compares a list of common antibicrobial compounds.
Antibiotic resistance is a significant concern due to the over use or inappropriate use of antibiotic therapy. Patients should be advised to complete their antibiotic therapy as prescribed to reduce the risk of antibiotic resistance. Selection of antibiotics should generally be made after wound swabs and sensitivity testing to determine the bacteria against which treatment should be directed(10).
Clinical indications that the patient has spreading infection (e.g. cellulitis) include(10):
Clinical indications that the patient has systemic infection (e.g. bacteraemia, sepsis) include(10):
All PIs should be assessed regularly for indicators of infection. For complex, unresponsive, recalcitrant or recurrent infection, consider consulting a microbiologist or infectious disease specialist(10).
Select a wound dressing. Wound dressings or devices are applied to a wound in order to protect the wound from contamination and trauma, to absorb exudate, to fill dead space deficits, reduce oedema and to promote an optimal healing environment. Wound healing is based on the principles of moist wound healing, which is optimised through dressings that donate fluid to the wound or the application of occlusive or semi- occlusive dressings, and wound bed preparation. All products should be used in accordance with the manufacturer’s directions(10).
Characteristics that are likely to influence wound dressing selection may include(10):
Continually moist gauze should be used only when other moisture retentive dressings are not available(10).
Negative pressure wound therapy (NPWT) is a wound management technique that involves application of suction to the wound using a vacuum dressing. It is reported to improve nutritional and oxygen delivery to the wound through reduction of oedema, to remove wound exudate, to promote tissue granulation, and to remove wound inhibitory factors. The therapy is primarily used to reduce wound volume and may be used to prepare the wound bed for flap closure surgery. A foam or gauze cavity filling dressing is used to fill the wound defect and sealed with a transparent film secondary dressing, through which a drainage tube connected to vacuum is inserted. When using TNPT(10):
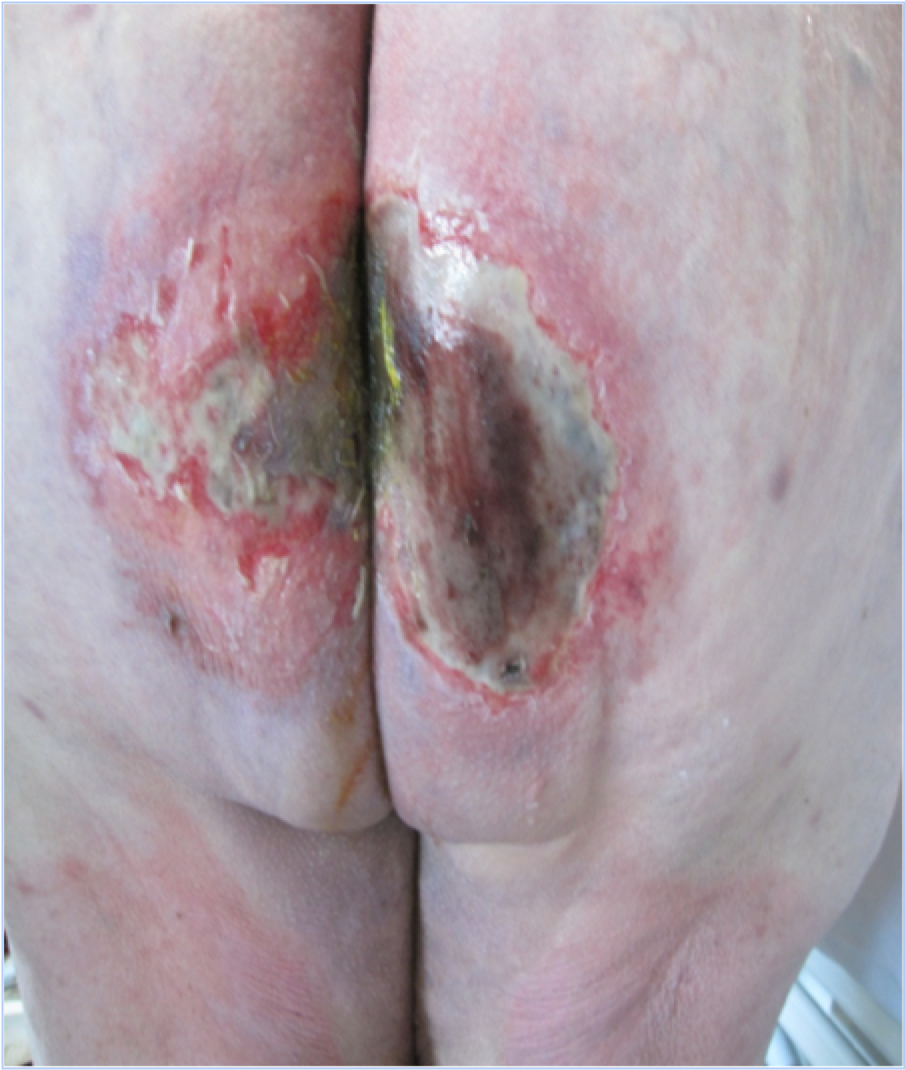
So if there is a link between the two, do we sometimes have to treat both?
Sometimes elements of both treatments will need to be used when there is a PI present within an area of IAD. The moisture that formed the IAD may have been a contributing factor in the formation of a PI, or alternatively, the exudate from the PI may have caused the maceration/excoriation associated with the dermatitis. Either way, just managing moisture or just removing pressure will not be effective and a combined management plan will need to be implemented(3).
| Pressure Ulcer | Moisture Lesion | Remarks | |
| Causes | Pressure and/or shear must be present. | Moisture must be present (eg, shining wet skin caused by urinary incontinence or diarrhea). | If moisture and pressure/shear are simultaneously present, the lesion could be a pressure ulcer as well as a moisture lesion (combined lesion). |
| Location | A wound not over a bony prominence is unlikely to be a pressure ulcer. | A moisture lesion may occur over a bony prominence. However, pressure and shear should be excluded as causes and moisture should be present. A combination of moisture and friction may cause moisture lesions in skin folds. A lesion that is limited to the anal cleft only and has a linear shape is not a pressure ulcer and is likely to be a moisture lesion.Perianal redness/skin irritation is most likely to be a moisture lesion resulting from faeces. | It is possible to develop a pressure ulcer where soft tissue is compressed (eg. By a nutrition tube, nasal oxygen tube or urinary catheter).Wounds in skin folds of bariatric patients may be caused by a combination of friction, moisture, and pressure.Bones may be more prominent where there is significant tissue loss (weight loss). |
| Shape | If the lesion is limited to one spot, it is likely to be a pressure ulcer.Circular wounds or wounds with a regular shape are most likely pressure ulcers; however, the possibility of friction injury has to be excluded. | Diffuse different superficial spots are more likely to be moisture lesions.In a kissing ulcer (copy lesion) at least one of the wounds is most likely caused by moisture (urine, faeces, transpiration, or wound exudate). | Irregular wound shapes are often present in a combines lesion (pressure ulcer and moisture lesion).Friction on the heels may also cause a circular lesion with full-thickness skin loss. The distinction between a friction lesion and a pressure ulcer should e made based on history and observation. |
| Depth | Partial-thickness skin loss is present when only the top layer of the skin is damaged (Stage II).In full-thickness skin loss, all skin layers are damaged (Stage III or IV).If there is a full-thickness skin loss and the muscular layer is intact, the lesion is a Stage III pressure ulcer. If the muscular layer is not intact, the lesion should be diagnosed as a Stage IV pressure ulcer. | Moisture lesions are superficial (partial-thickness skin loss).In case where the moisture lesions gets infected, the depth and extent of the lesion can be enlarged/deepened extensively. | An abrasion is caused by friction.If friction is exerted on a moisture lesion, this will result in superficial skin loss in which skin fragments are torn and jagged. |
| Necrosis | A black necrotic scab on a bony prominence is a pressure ulcer (unstageable).Necrosis can also be considered present at the heel when the skin is intact and the black/blue shimmer is visible under the skin (deep tissue injury-the lesion will most likely resolve into a necrotic eschar) | There is no necrosis in a moisture lesion. | Necrosis starts without a sharp edge but evolved into sharp edges. Necrosis softens up and changed colour (eg, blue, brown, yellow, or grey) but is never superficial.Distinction should be made between a black necrotic scab and a dried blood blister. |
| Edges | If the edges are distinct, the lesion is most likely a pressure ulcer.Wound with raised and thickened edges are old wounds. | Moisture lesions often have diffuse or irregular edges. | Jagged edges are seen in moisture lesions that have been exposed to friction. |
| Colour | Red skin:If redness is non-blanchable, this is most likely a pressure ulcer Stage I.For people with darkly pigmented skin, persistent redness may manifest as blue or purple.Red in wound bed:If there is red tissue in the wound bed, the wound is either a Stage II, a Stage III or a Stage IV pressure ulcer with granulation tissue in the wound bed.Yellow in wound bed:Softened necrosis is yellow and not superficial.Slough is a creamy, thin and superficial layer.Black in the wound bed:Black necrotic tissue in the wound bed indicates a pressure ulcer. | Red skin:If the redness is not uniformly distributed, the lesion is likely to be a moisture lesion (exclude pressure and shear as causes).Pink or white surrounding skin:Maceration resulting from moisture. | Red skin:If the skin (or lesion) is red and dry or red with a white sheen, it could be a fungal infection or intertrigo.This is often observed in the anal cleft.Green in wound bed: Infection.Be aware that zinc oxide ointments may result in whitened skin.While eosin is not recommended, it is still used in some areas. It will turn the skin red/brown and obstruct the observation of the skin. |
Modified from Pressure Ulcer Advisory Panel’s “Differentiation Between Pressure Ulcers and Moisture Lesions”(1)
| Type | Mechanisms of action | Advantages | Disadvantages | Who/where |
| Autolytic | Uses the body’s own enzymes and moisture to rehydrate, soften and liquefy hard eschar and slough using occlusive or semi-occlusive dressings and/or antimicrobial products to create a balanced moist wound environment either by donating or absorbing moisture | Can be used for pre-debridement, when there is a small amount of non-viable tissue. Also suitable for wounds where other forms of debridement are inappropriate.Can be used for maintenance debridement | The process is slow, increasing potential for infection and maceration | Can be done by both generalist and specialist |
| Enzymatic | Uses enzymes to break down a number of inorganic and organic compounds to remove eschar and slough. Requires a secondary dressing. Both Honey and Flamminal have antimicrobial properties. | Suitable for wounds where other forms of debridement are inappropriate.Can be used for maintenance debridement | High levels of exudate can damage periwound skin. Steps must be taken to prevent periwound maceration and excoriation. | Can be done by both generalist and specialist |
| Mechanical | Traditional method involves using wet to dry gauze that dries and adheres to the top layer of the wound bed, which is ‘pulled’ away when the dressing is removed | Newer methods are more selective, faster and relatively pain-free. | Non-selective and traditional methods are potentially harmful Requires frequent dressing changes and can be very painful for the patient | Can be done by both generalist and specialist |
| Biosurgical | Larvae of the green bottle fly are used to remove necrotic and devitalised tissue from the wound. Larvae are also able to ingest pathogenic organisms in the wound. | Highly selective and rapid | Costs are higher than autolytic debridement, but treatment is short once in place. Not suitable for all patients or wounds | Can be applied by generalist or specialist practitioner with training. Closed bag method reduces skill level required and can be left for 4-5 days |
| Hydrosurgical | Removal of dead tissue using a high energy saline beam as a cutting implement | Short treatment time and selective. Capable of removing most if not all devitalised tissue from the wound bed | Requires specialist equipment. There is potential for aerosol spread and it is associated with higher costs | Must be carried out by a specialist practitioner with relevant training. Can be used in a variety of settings. |
| Sharp | Removal of dead or devitalised tissue using a scalpel, scissors and/or forceps to just above the viable tissue level. This does not result in total debridement of all non-viable tissue and can be undertaken in conjunction with other therapies (eg autolysis) | Selective and quick. No analgesia is required normally | Clinicians need to be able to distinguish tissue types and understand anatomy as the procedure carries the risk of damage to blood vessels, nerves and tendons | Can be done at the patient’s bedside or in clinic by a skilled practitioner with specialist training |
| Surgical | Excision or wider resection of non-viable tissue, including the removal of healthy tissue from the wound margins, until a healthy bleeding wound bed is achieved | Selective and is best used on large areas where rapid removal is required | It can be painful for the patient and anaesthetic is normally required. It can be associated with higher costs | Must be performed in the operating theatre by a surgeon, podiatrist or specialist nurses following training |
| Ultrasonic | Devices deliver ultrasound either in direct contact with the wound bed or via an atomised solution (mist). Most devices include a built-in irrigation system and are supplied with a variety of probes for different wound types | Immediate and selective. It can be used for excisional debridement and/or maintenance debridement over several sessions | Availability issues due to higher costs and requirement for specialist equipment. Requires longer set up and clean up time (involving sterilisation of hand pieces) than sharp debridement. | Must be carried out by competent practitioner with specialist training in a variety of settings |
This table has been copied directly from “Debridement made easy”(15).
| Product | Method of Action | Effect on wound healing | Indication/contraindication | references |
| Cadexomer Iodine (Iodosorb - microspherical beads of hydrophilic biodegradable starch and 0.9% (w/v) iodine) |
|
|
| (16-21) |
| Silver - Nanocrystaline silver such as Acticoat or as Ionic Silver combined with many dressing types including, but not limited to, hydrofibres (Aquacel) and foams (Mepilex Ag) |
|
|
| (22-24) |
| Honey (Manuka Honey, Medihoney) |
|
|
| (19, 25-31) |
| Hypertonic Saline - Curity: 20% solution of NaCl on a gauze dressing, can be sheet or ribbon (previously Curasalt) |
|
|
| (32) |
| Chlorhex impregnated gauze - Bactigras |
|
|
| (23, 33, 34) |
| Prontosan Solution (Polyhexamethylene biguanide (PHMB) (0.1%) and Betaine a surfactant (0.1%)) |
|
|
| (23, 35) |
1. Defloor, T., et al., Statement of the European Pressure Ulcer Advisory Panel -- pressure ulcer classification: differentiation between pressure ulcers and moisture lesions... including commentary by Doughty D. Journal of Wound, Ostomy & Continence Nursing, 2005. 32(5): p. 302-306.
2. Beeckman, D., et al., Pressure ulcers and incontinence-associated dermatitis: effectiveness of the Pressure Ulcer Classification education tool on classification by nurses. Quality & Safety In Health Care, 2010. 19(5): p. e3-e3.
3. Corcoran, E. and S. Woodward, Incontinence-associated dermatitis in the elderly: treatment options. British Journal Of Nursing, 2013. 22(8): p. 450-457.
4. Denat, Y. and L. Khorshid, The effect of 2 different care products on incontinence-associated dermatitis in patients with fecal incontinence. Journal of Wound, Ostomy & Continence Nursing, 2011. 38(2): p. 171-176.
5. Bliss, D.Z., et al., Incontinence-associated dermatitis in critically ill adults: time to development, severity, and risk factors. Journal Of Wound, Ostomy, And Continence Nursing: Official Publication Of The Wound, Ostomy And Continence Nurses Society / WOCN, 2011. 38(4): p. 433-445.
6. Beeckman, D., et al., A 3-in-1 Perineal Care Washcloth Impregnated With Dimethicone 3% Versus Water and pH Neutral Soap to Prevent and Treat Incontinence-Associated Dermatitis: A Randomized, Controlled Clinical Trial. Journal of Wound, Ostomy & Continence Nursing, 2011. 38(6): p. 627-634.
7. Baadjies, R., I. Karrouze, and K. Rajpaul, Using no-rinse skin wipes to treat incontinence-associated dermatitis. British Journal Of Nursing, 2014. 23: p. S22-8.
8. Beeckman, D., et al., A Systematic Review and Meta-Analysis of Incontinence-Associated Dermatitis, Incontinence, and Moisture as Risk Factors for Pressure Ulcer Development. Research in Nursing & Health, 2014. 37(3): p. 204-218.
9. National Pressure Ulcer Advisory Panel & European Pressure Ulcer Advisory Panel. (2009). Pressure Ulcer Treatment: Technical Report, from http://www.npuap.org
10. The Australian Wound Management Association (AWMA) Pan Pacific Guideline Development Steering Committee, Pan Pacific Clinical Practice Guideline for the Prevention and Management of Pressure Injury.2012, Osborne Park, WA: Cambridge Media.
11. Hermans, M.H.E. and T. Treadwell, An Introduction to Wounds, in Microbiology of Wounds, S. Percival and K. Cutting, Editors. 2010, CRC Press: Boca Raton, Fla. p. 83-134.
12. Sprigle, S. and S. Sonenblum, Assessing evidence supporting redistribution of pressure for pressure ulcer prevention: A review. Journal of Rehabilitation Research & Development, 2011. 48(3): p. 203-213.
13. Bluestein, D. and A. Javaheri, Pressure ulcers: prevention, evaluation, and management. American Family Physician, 2008. 78(10): p. 1186-1194.
14. European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel, Treatment of pressure ulcers: Quick Reference Guide2009, Washington DC: National Pressure Ulcer Advisory Panel.
15. Vowden, K. and P. Vowden, Debridement made easy. Wounds UK, 2011. 7(4): p. 1-4.
16. Tatro, D.S. and L.R. Borgsdorf, A to Z drug facts, ed. D.S. Tatro2005, St. Louis, Mo. ; [Great Britain]: St. Louis, Mo. ; Great Britain : Facts & Comparisons.
17. Percival, S.L., R.A. Cooper, and B.A. Lipsky, Chapter 11. Antimicrobial Interventions for Wounds, in Microbiology of Wounds, S. Percival and K. Cutting, Editors. 2010, CRC Press: Boca Raton, Fla.
18. O'Meara, S., et al., Antibiotics and antiseptics for venous leg ulcers, 2010, Cochrane Database of Systematic Reviews.
19. Bowler, P.G., B.I. Duerden, and D.G. Armstrong, Wound microbiology and associated approaches to wound management. Clinical Microbiology Reviews, 2001. 14(2): p. 244-269.
20. Panuncialman, J. and V. Falanga, The science of wound bed preparation. Surgical Clinics of North America, 2009. 89(3): p. 611-626.
21. O'Toole, E.A. and J.E. Mellerio, Chapter 14. Wound Healing, in Rook's Textbook of Dermatology, T. Burns, et al., Editors. 2010, Wiley-Blackwell: Oxford, UK.
22. Hampton, S., How and When to Use Antimicrobial Dressings. Nursing and Residential Care, 2010. 12(11): p. 522-529.
23. Percival, S.L., R.A. Cooper, and B.A. Lipsky, Antimicrobial Interventions for Wounds, in Microbiology of Wounds, S. Percival and K. Cutting, Editors. 2010, CRC Press: Boca Raton, Fla. p. 293-327.
24. Tonkin, C. and F. Wood, Nanocrystalline silver reduces the need for antibiotic therapy in burn wounds. Primary Intention: The Australian Journal of Wound Management, 2005. 13(4): p. 163-168.
25. White, R., Chapter 12. Wound Dressings and Other Topical Treatment Modalities in Bioburden Control, in Microbiology of Wounds, S. Percival and K. Cutting, Editors. 2010, CRC Press: Boca Raton, Fla.
26. Glasser, J.S., et al., Activity of topical antimicrobial agents against multidrug-resistant bacteria recovered from burn patients. Burns, 2010. 36(8): p. 1172-1184.
27. Leon-Villapalos, J., M.G. Jeschke, and D.N. Herndon, Topical management of facial burns. Burns, 2008. 34(7): p. 903-911.
28. Benbow, M., Fungating malignant wounds and their management. Journal of Community Nursing, 2009. 23(11): p. 12.
29. Hampton, S., How and When to Use Antimicrobial Dressings. Nursing & Residential Care, 2010. 12(11): p. 522-529.
30. Robson, V., S. Dodd, and S. Thomas, Standardized antibacterial honey (Medihoney[TM]) with standard therapy in wound care: randomized clinical trial. Journal of Advanced Nursing, 2009. 65(3): p. 565-575.
31. Jull, A.B., A. Rodgers, and N. Walker, Honey as a topical treatment for wounds. Cochrane Database Of Systematic Reviews (Online), 2008(4): p. CD005083.
32. Leaper, D.J., et al., Extending the TIME concept: what have we learned in the past 10 years. International Wound Journal, 2012. 9 (Suppl. 2): p. 1-19.
33. Owens, B.D., D.W. White, and J.C. Wenke, Comparison of irrigation solutions and devices in a contaminated musculoskeletal wound survival model. Journal of Bone and Joint Surgery, 2009. 91(1): p. 92-98.
34. Vali, L., et al., Frequency of biocide resistance genes, antibiotic resistance and the effect of chlorhexidine exposure on clinical methicillin-resistant Staphylococcus aureus isolates. The Journal Of Antimicrobial Chemotherapy, 2008. 61(3): p. 524-532.
35. Bradbury, S. and J. Fletcher, Prontosan made easy. Wounds International, 2011. 2(2): p. s25-s30.
© 2015 Wound Care Resource All rights reserved | Web template modified from W3layouts